Many of the proteins that play a crucial role in living cells adhere to a core principle of biology: their form, or shape, fits their function. But there is also a vast number of proteins and their parts that defy that dogma.
Why it matters: New findings are revealing how these flexible, disordered proteins work — and deciphering their role in human diseases and potential treatments.
How it works: Whether many medicines, immune cells, or the moment-to-moment inner workings of cells function depends on the shape of proteins they interact with or use.
- "The classical picture of proteins is they are molecular machines," says Alex Holehouse, a professor at Washington University in St. Louis. They have certain parts set in certain positions and "their function depends on how those parts move together."
But the other 40% of the proteins made in the human body are different.
- Instead of having a specific three-dimensional structure, these intrinsically disordered proteins (IDP) or intrinsically disordered protein regions (IDPR) each have a collection of thousands of possible distinct shapes, or ensemble.
- "A lot of the conceptual or intellectual tools that you would use to think about protein function are no longer applicable," Holehouse says.
- And, a lot of the tools used to determine a protein's structure also can't be used on disordered proteins. The gold standard method is to crystallize a protein and then study it with X-rays — a time- and cost-consuming method. New machine-learning systems, including AlphaFold2 and RoseTTAFold, are able to predict structures much faster.
Disordered proteins present their own challenges for these systems. But DeepMind is studying how they bind to other proteins — a "core function of disordered proteins," John Jumper, who leads the AlphaFold2 team at DeepMind, tells Axios. That information can help to determine what structure the proteins may adopt.
- Other researchers are also using AlphaFold2 to study the multiple states of some disordered proteins.
Background: Biologists have studied disorder proteins for more than three decades. At first, it was thought that without a specific structure, they couldn't exist and would be chopped up by enzymes in cells.
- But then they were found and determined to have a key role in fundamental processes in the cell, including the mechanisms that transcribe DNA into RNA.
- "Their disorder is key to their function," says Peter Wright, a professor at Scripps Research Institute who developed methods in the 1990s to determine the structure of disordered proteins. Many proteins have both disordered and ordered regions and "the synergy between them is critical."
- Then these curious proteins were found to be involved in forming compartments in cells that don't have walls or membranes, but where molecules involved in repairing DNA or synthesizing RNA and translating it into proteins concentrate. These condensates, which also involve structured proteins, are an intense focus of cellular biologists today.
- Disorder proteins are also implicated in diseases ranging from cancer to cardiovascular disease to neurodegeneration. But it has been unclear how.
What's new: A paper published this week in the journal Nature found that a mutation in a disordered region of a protein may direct the protein to the wrong condensate.
- Once there, the protein essentially "poisons" the condensate, says Denes Hnisz, a co-author of the new study, and researcher at the Max Planck Institute for Molecular Genetics in Berlin.
What they did: Hnisz and his colleagues looked at a genetic mutation found in patients with an extremely rare genetic disease called BPTA syndrome which results in malformations in a person's limbs, face, and bone and nervous system.
- Five of the less than 10 people in the world known to have BPTA syndrome took part in the study.
- The researchers found the mutation changed the charge of the tail of a particular disordered protein (HMGB1) from positive to negative. This tail acts as bar code that partitions the protein to the right place in the cell.
- That alteration meant the mutated protein was then attracted to the nucleolus — a condensate in the cell nucleus — that became solidified as a result. In tests in a laboratory dish, more of the cells that had the mutated protein died than those that didn't have it.
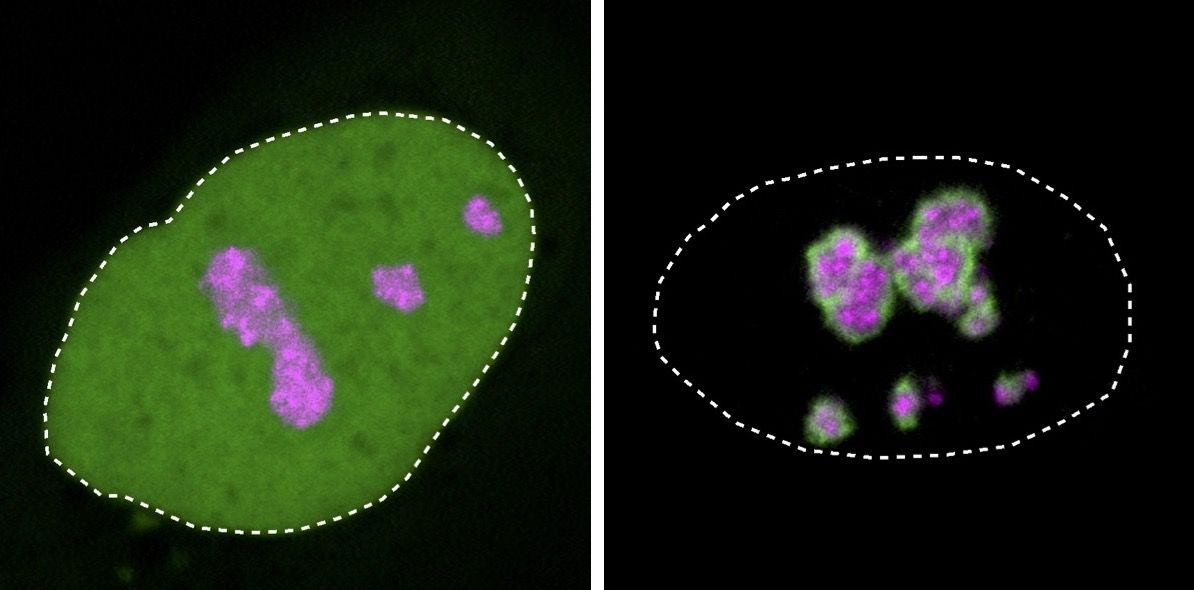
When Hnisz and his colleagues searched genomic databases, they found more than 600 similar mutations in 66 different proteins that caused a change in the charge of a protein's tale — and 101 are associated with other disorders.
- They then looked at 13 mutant genes and the proteins they created. When they tested them in the lab, 12 were sent to the nucleolus, which stopped functioning with about half of the proteins.
- That suggests the mechanism could be at work in other diseases, the researchers write.
What they're saying: The findings are "a huge conceptual leap" for the field, says Keren Lasker, a professor at Scripps Research Institute who studies condensates in bacteria and wasn't involved in the research.
- A big question has been what is the functional role of condensates, she says.
- "Here we see it is putting the right proteins in the right place at the right time for the right developmental patterns to occur."
- The work provides new insights about the "amazing number of mutations that we didn’t know what to make of," says Rohit Pappu, a professor at Washington University in St. Louis who studies disordered proteins but wasn't involved in the study. It suggests they're "rewriting the IDPR grammar, which means they are rewriting what they do and where they go."
The intrigue: The discovery won't help with treatments for BPTA, a congenital disease. But Hnisz says disordered proteins may provide clues about designing therapeutics for other diseases, particularly cancer.
- Other researchers, including Holehouse, are investigating the role of disordered proteins in an organism's ability to withstand extreme environments.
- About 5% of the proteins in bacteria are disordered. They use them "in a way that is somehow similar but also different from how mammalian cells are using them," says Lasker, whose lab works on engineering synthetic condensates that operate as "garbage collectors" in human cells in the lab.
- "Disorder proteins are just an amazingly rich source of research ideas," Hnisz says.







