
Two drugs to treat Alzheimer’s disease have been rejected for use on the NHS because their benefits are “too small” to justify their cost, the health spending watchdog has said.
Donanemab and lecanemab are targeted antibody drugs that slow down the early stages of Alzheimer’s by targeting a known cause, rather than just treating symptoms.
Both drugs bind to amyloid, a protein which builds up in the brains of people living with Alzheimer’s disease, and are designed to help clear the build-up and slow down cognitive decline.
However, in publishing its final draft guidance, the National Institute for Health and Care Excellence (Nice) said the treatments have been shown to delay progression from mild to moderate Alzheimer’s by four to six months, which it described as only “modest benefits at best”.
What are the medicines?
Donanemab and lecanemab are targeted antibody drugs that slow down the early stages of Alzheimer’s.
They represent a huge step forward in research because they target a known cause of the disease, rather than just treating the symptoms.
Both drugs bind to amyloid, a protein which builds up in the brains of people living with Alzheimer’s disease.
By binding to amyloid, the drugs are designed to help clear the buildup and slow down cognitive decline.
How effective are they?
Donanemab has been shown in clinical trials to slow the rate at which memory and thinking get worse by more than 20 per cent.
Evidence suggests that people get the most benefit if they are given the treatment earlier in the disease.
Results also suggest the drug leads to a 40 per cent slowing in the decline of everyday activities such as driving, enjoying hobbies and managing money.
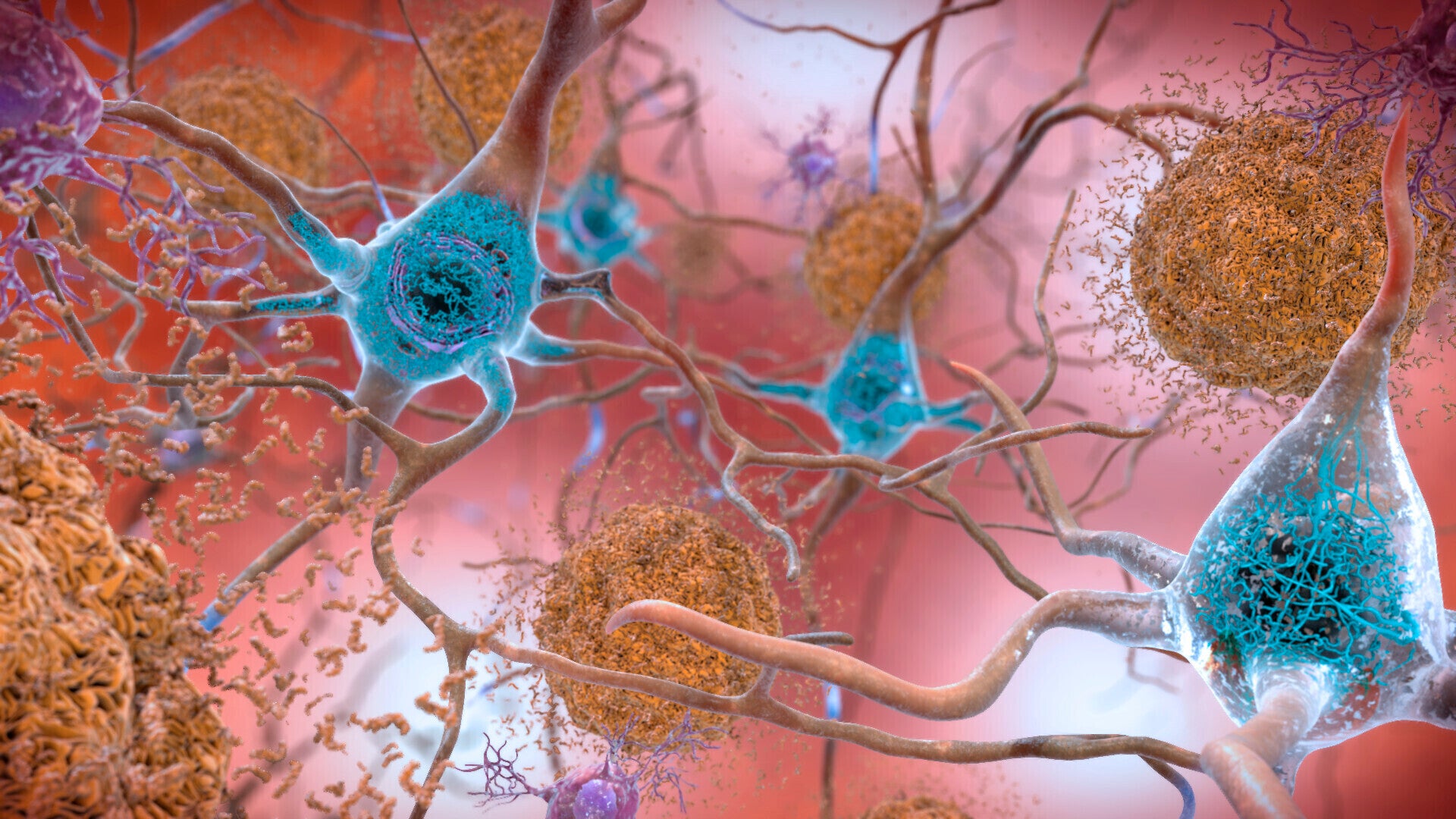
Lecanemab has also been shown to successfully remove protein build-up from the brains of people living with early Alzheimer’s disease.
For people taking lecanemab, this meant the decline in their thinking and memory skills was slowed down by 27 per cent.
It also slowed down the decline in quality of life by up to 56 per cent.
How are the drugs given?
Donanemab, developed by the pharmaceutical company Lilly, is given to patients via an intravenous drip once every four weeks.
Lecanemab, developed by Eisai, is also given this way, but fortnightly.
Are there any side effects?
Side effects of the drugs can be serious, and people undergo monitoring to check for them.
In one clinical trial published in the Journal of the American Medical Association (JAMA) in 2023, 24 per cent of people receiving donanemab had side effects, including brain swelling and infusion-related reactions.
Four people died during the trial, with their deaths thought to be related to the drug’s side effects.

Lecanemab resulted in infusion-related reactions in around 26 per cent of people on the trial and followed up, while 14 per cent suffered amyloid-related imaging abnormalities, causing brain swelling. Others suffered minor bleeds, picked up on scans.
Around one in 10 people suffered headaches, according to updated results published in May 2024.
Overall, four deaths during the follow-up period were thought to be due to treatment.
How much do the drugs cost?
NHS England published a briefing paper last year suggesting the cost of bringing the drugs to the health service could be £500 million to £1 billion per year.
Around 50 per cent to 60 per cent of the total estimated cost relates to the drug price, with the remaining cash spent on patient assessment, diagnosis and administering the treatment.
How many people in England might the drugs have worked for?
NHS England estimated that between 50,000 and 280,000 patients could be eligible for the new treatments if they were approved for the NHS.
To get the drugs, patients need a baseline MRI scan and then either a PET-CT scan or lumbar puncture to confirm Alzheimer’s.
It is possible that blood tests will be available in the future to diagnose the disease, so NHS England did warn there should be caution about driving a “massive expansion” in other diagnostics, which could become redundant in the longer term.
What do scientists think?
Scientists and doctors have been divided on whether the drugs represent a real clinical benefit that is noticeable in patients' day-to-day.
Some argue the drugs represent a huge advance, and people should be given the chance to try them.
But others say the benefits are too small.

Jennifer Keen, associate director of evidence, policy and influencing at the Alzheimer’s Society, has said that “we remain at an important and exciting moment”.
“There are currently 182 active clinical trials for Alzheimer’s disease… We are on the cusp of major scientific breakthroughs beginning to improve the outlook for those with the disease.”
Professor Rob Howard, from University College London, said that “nobody should be surprised that Nice have confirmed their earlier view that the new Alzheimer’s disease treatments would not be cost-effective if used within the NHS.
“Well-conducted clinical trials demonstrated that the actual size of benefits experienced by patients were too small to be noticeable, treatment carries risks of side-effects, and the annual cost of the drugs and safety monitoring required would have been close to the cost of a nurse’s salary for each treated patient.
“We need better treatments that can make an appreciable difference to the lives of people with dementia and these can only come from further research and study.”
Thousands of NHS patients denied breakthrough Alzheimer’s drugs
How do the new antibody drugs for Alzheimer’s disease work?
‘Pioneering’ technology to be used on UK brain tumour patients in world-first
Imperial beats Oxbridge in global university rankings for second year in a row
Scandal-hit nursing regulator wrongly cleared 350 nurses to work in UK
Toxic air pollution could be linked to 30,000 deaths in the UK this year







