A New Jersey-based pharmaceutical company issued a voluntary recall for over half a million bottles of Prazosin Hydrochloride after clinical tests revealed that the capsules contain high levels of potentially cancer-causing chemicals.
The Food and Drug Administration released an enforcement report to announce that Teva Pharmaceuticals USA Inc. recalled a total of 580,844 bottles of blood pressure medication, commonly known as Prazosin Hydrochloride, which were distributed around the country.
NBS Chicago revealed that based on the report, the pharmaceutical company decided to recall the capsules, which were sold in doses of 1, 2, and 5 mg, because they were detected with 'above acceptable limits' for N-nitroso Prazoin impurity C, a chemical compound linked to cancer.
Teva Pharmaceuticals initiated the recall on 7 October, then classified it as a 'Class II' on 24 October, meaning the product 'may cause temporary or medically reversible adverse health consequences' or the possibility of serious adverse health effects is slim.
According to the California Board of Pharmacy memo, 'The overall risk of harm in the patient population is considered to be medium.'
What is Prazosin Hydrochloride?
The Prazosin Hydrochloride capsules are a type of medication prescribed to treat high blood pressure.
It works by relaxing the blood vessels and allowing the blood to pass through easily all over the body.
While the drug is being prescribed to cure high blood pressure, several studies also claimed that it can also be used to treat post-traumatic stress disorder (PTSD) symptoms.
High blood pressure can be easily managed, even if other people say otherwise.
Those who have high blood pressure should incorporate a healthy lifestyle. They must also have a healthy and balanced diet, regular exercise, and maintain an ideal weight.
It is also best to avoid eating sugary and salty medicines, including cheese and donuts. It is also best to avoid drinking too much alcohol, smoking, and drinking too much caffeine.
High Blood Pressure Defined
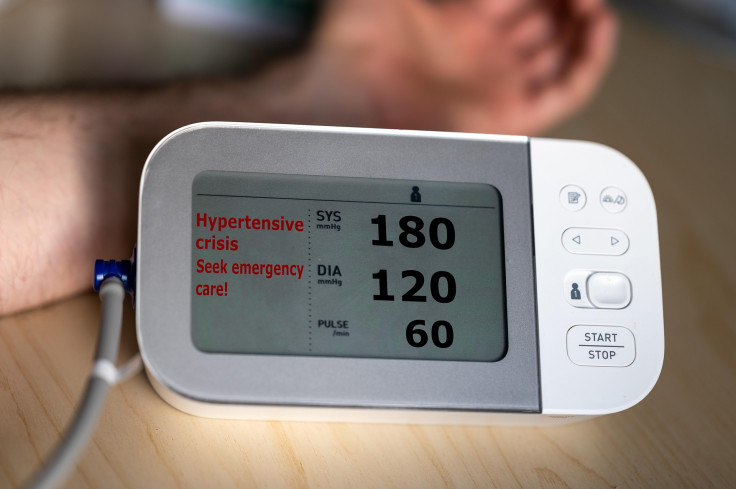
NHS shared that high blood pressure, also known as hypertension, is a very common lifestyle disease, especially in adults.
Those who have high blood pressure do not feel any symptoms, but it can sometimes bring headaches, chest pain, and blurred vision.
Since it rarely shows any symptoms, the best way to find out if a person has hypertension is to have blood pressure checked.
Those who have high blood pressure will be at risk of having other conditions, including heart disease, cardiovascular attacks, strokes, kidney disease, heart failure, or vascular dementia.
Which Bottles are Included in the Recall?
Teva Pharmaceuticals revealed that the Prazosin Hydrochloride recall affects 181,659 bottles containing 1 mg capsules, 291,512 bottles of 2 mg capsules, and 107,673 bottles of 5 mg capsules.
The FDA and Teva did not release any guidance on what to do with the bottles of recalled medicine.
According to guidance from GoodRx, individuals affected by a drug recall should first check the lot number on their medication bottle. If it matches the recalled batch, they should contact their pharmacist and the prescribing doctor for advice. Once confirmed, the medication should be safely disposed of following local pharmacy or health authority recommendations.
Get Help for Hypertension
The NHS offers a range of free support services to help individuals manage high blood pressure and improve overall health. These include tools and programs to assist with weight loss, quitting smoking, and reducing alcohol consumption. By adopting healthier habits, people can significantly lower their risk of hypertension-related complications.