HYDERABAD: The World Health Organisation (WHO) is on track to take a decision on the Emergency Use Listing of India’s first indigenously developed Covid-19 vaccine Covaxin in October.
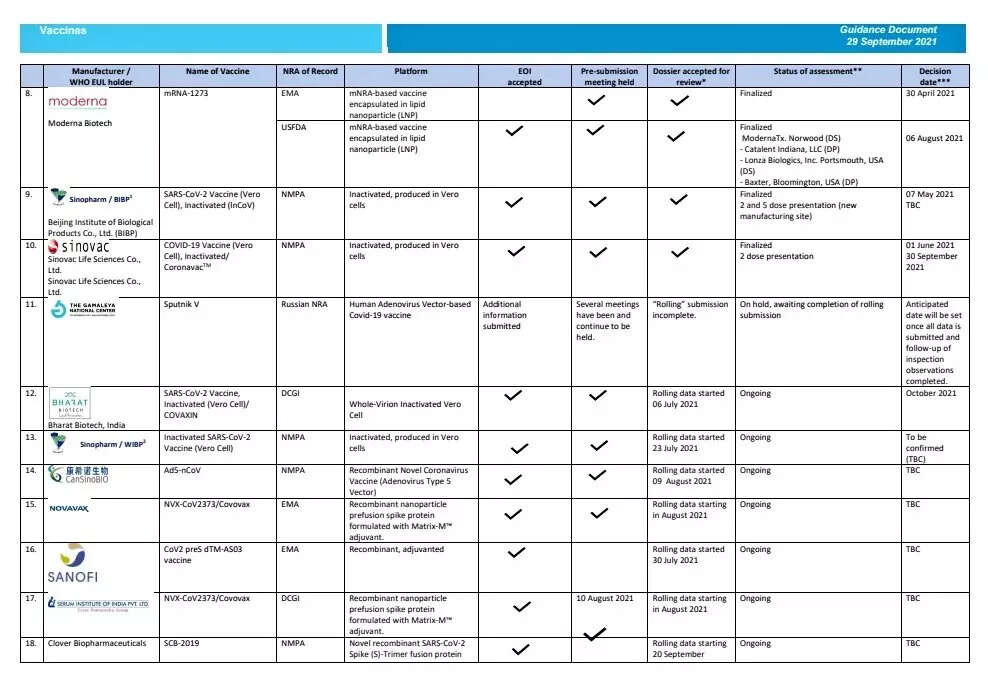
The global health body has updated the decision date for Bharat Biotech’s Covaxin as October 2021 on its latest EUL guidance document for Covid-19 vaccines dated September 29. Prior to this latest update, the status on the WHO website for the vaccine showed as “yet to be confirmed”.
While the Covaxin assessment status shows as ongoing on the WHO website, the Strategic Advisory Group of Experts on Immunisation (SAGE) of WHO will be meeting on October 5 to discuss and consider for recommendation the EUL for Covaxin.
As per the draft agenda of the SAGE meeting uploaded on WHO’s website, Bharat Biotech will be making a presentation to the expert group on the safety and efficacy data of the vaccine’s Phase-1, 2 and 3 clinical trial results along with the risk management plans and other implementation considerations.
During the meeting SAGE will discuss the Covaxin clinical trials data from phase 1, 2, 3 and post marketing studies on safety, immunogenicity, efficacy and effectiveness as well as the outline of ongoing and planned studies on its safety and effectiveness along with update on global, regional and country level plans for vaccine safety monitoring.
After the group assesses the evidence, SAGE member Hanna Nohynek will present the draft recommendation for the vaccine. “Based on the presented evidences, the expert panel will present its draft recommendations on the use of Bharat Biotech’s vaccine in priority populations,” the SAGE agenda said.
SAGE, which is the principal advisory group to WHO for vaccines and immunization, advises WHO on overall global policies and strategies, ranging from vaccines and technology, research and development, to delivery of immunization and its linkages with other health interventions.
Covaxin’s EUL has been under review by WHO since July 6 when the rolling data submission started. Many of those who have taken the jab have been awaiting a WHO nod as it would make international travel easier.


.jpg?w=600)




