
Guilherme – June 16, 2020. Evening TV news shows across the world were suddenly opening with the result of our research, and rightly so. Dexamethasone was the first drug to be shown to save the lives of people infected with COVID-19. To a young researcher like me, that day felt like being part of history. It was a gift from the UK to the world.
Two years ago this week, the Recovery trial transformed the care of COVID patients with its dexamethasone announcement. Within four hours, the steroid was included in NHS treatment recommendations. Almost overnight, treatment of COVID patients around the world changed completely. It has been estimated that dexamethasone may have saved a million lives in the first nine months following the announcement.
Recovery, jointly led by Oxford Population Health and the Nuffield Department of Medicine, is a groundbreaking scientific machine which, from the outset, moved at unprecedented speed. Within 15 days, more than 1,000 participants around the UK had joined the trial; five weeks later, that number had risen to 10,000. In the first 100 days alone, the trial produced three groundbreaking results that would completely reshape COVID care.
From an impromptu discussion between two professors on a London bus to headlines around the world – and a baby being born at home in the midst of the pandemic – this is the inside story of Recovery, by three medics who have been part of this extraordinary journey.
The vast and unique nature of the NHS provided the perfect setting for the trial’s rapid development. Indeed, if a similar approach could now be employed for other common and important diseases, we believe it could transform the quality of evidence supporting treatments for millions more people in the UK and around the world.
How it all began
Mark – Working on hospital wards during the first wave of the pandemic was at times a haunting experience. Patients of all ages were severely ill, and could deteriorate rapidly with little warning. I spent many hours talking to patients at their bedside and to their relatives on the phone – not knowing when the last conversation might be; wanting to offer hope but not false hope. The emotional toll was profound, but the privilege of being there for patients and their families at such a terrifying time was enormous.
Early March 2020. Despite growing concerns about the worldwide spread of COVID-19, life in the UK continued pretty much unchanged. Daily commuting, large sporting events and international travel all kept going, even after the virus began circulating within the UK and the first deaths occurred.
But in scientific circles, discussions were already under way about how to respond to this mysterious virus. Following a call for research proposals in early February, public funding for a UK-based trial was agreed on March 5.
Four days later Professor Martin Landray, an experienced clinical trialist based at Oxford Population Health, and Sir Jeremy Farrar, director of Wellcome, happened to meet on a No.18 London bus journey. Both agreed that a major public health crisis was looming.
“What we agreed on that bus trip was that the tsunami would arrive within a fortnight,” Landray later recalled. “So we had to have the trial up and running within two weeks.” On March 11, the World Health Organization declared COVID-19 a global pandemic, and on March 23, the UK imposed its first national lockdown.

This story is part of Conversation Insights
The Insights team generates long-form journalism and is working with academics from different backgrounds who have been engaged in projects to tackle societal and scientific challenges.
Landray joined forces with Professor Peter Horby, a specialist in emerging infectious diseases at the Nuffield Department of Medicine. They recognised that COVID-19 was an entirely new disease for which no proven treatments were available. Many were making educated guesses regarding what did and didn’t work, resulting in a jigsaw of different clinical recommendations around the world, but no one knew much for certain.
And so Recovery (short for the Randomised Evaluation of COVID-19 Therapy) was established to embrace, rather than deny, these uncertainties – and to harness the best scientific methods to resolve them, rather than relying on hype or hope.
But this mantra did not land well with some people, who mistook scientific rigour and the quest for definitive answers for dangerous delaying tactics, thinking they already knew which treatments would and wouldn’t work. As Horby later recalled: “I’ve got a drawer full of letters telling me I’m killing people.”
The guiding principles of Recovery
Leon – I was working on the Infectious Diseases ward at Oxford’s John Radcliffe Hospital in March 2020 when I first heard about the Recovery trial. In over a decade working in hospital medicine, I had never known a trial that felt accessible to normal clinicians, but Recovery was different. Involvement of medical teams was encouraged and made easy.
Already, various drugs had been proposed as treatments for COVID-19, but there was no good evidence that any of them actually helped. We knew that by randomising participants, we would discover if any of the drugs being tested actually did anything. In those early days, I remember that many of us thought dexamethasone was the least promising – until June 2020, when we were delighted to be proved wrong.
To be successful, Recovery needed a mechanism for conducting randomised controlled trials at a scale large enough to provide conclusive evidence. Unless the number of patients involved in such a trial is large, the play of chance can mean sicker patients are more common in one group, masking any effect of the treatment being tested (in the same way you need to toss lots of coins for the number of heads and tails to be reliably balanced).
Thousands of patients had to be enlisted as soon as possible. This was no trivial task – especially given the immediate impact of the pandemic on clinical research due to staff shortages and redeployment. Healthcare workers were under unprecedented strain, and there was little room for additional research commitments.
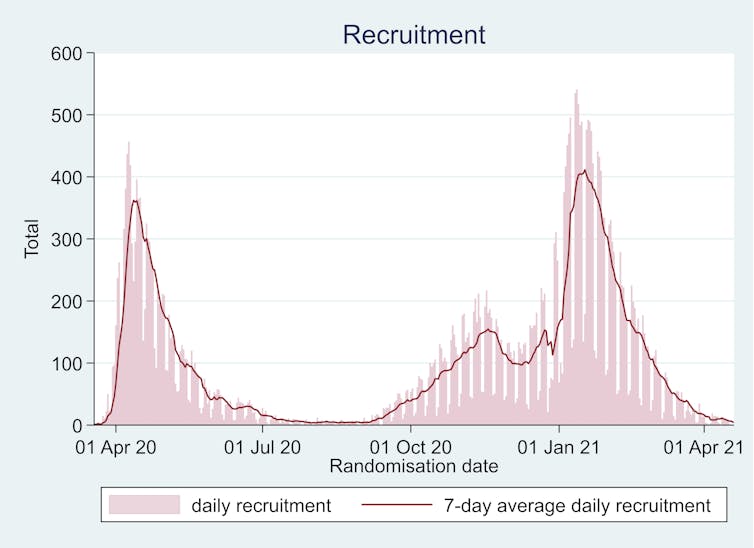
To achieve the scale required, Recovery had to be simple, making it easy for busy frontline staff to enlist unwell patients, while at the same time adhering to high scientific and ethical standards. The trial achieved this with a disruptive yet beautifully streamlined design. In part, it relied on clinical trial methodology first used in the 1980s – a highly pragmatic approach, focused on collecting small amounts of data on large numbers of people.
But it also made use of the latest available data technology – notably, the NHS’s healthcare data collection system. Much of the data generated as part of routine NHS care is gathered centrally to help allocate resources to hospitals and disease surveillance. This information can be quickly repurposed to help scientists run clinical research, and has been instrumental in the UK’s fight against COVID-19.
Professors Landray and Horby put together a small team in Oxford, drafted a protocol and submitted it to the research regulators. On March 19 – less than a fortnight after funding had been agreed – the first patient entered the Recovery trial. The speed at which this occurred was in itself a major breakthrough from traditional clinical trials, which typically take months (and sometimes years) to take off.
Every day the trial got bigger as more hospital sites were included. By the end of spring 2020, every single acute hospital in the UK – 176 in total – was a recruiting Recovery site. Now every person admitted to a hospital with COVID-19 could be asked to participate in the trial. Within 12 weeks, Recovery had grown into a truly national endeavour.
Why the structure of the NHS was key
Guilherme – I became involved in Recovery in April 2020. As a junior investigator working in clinical trials, my main role was to provide remote support to frontline staff recruiting ill patients. I was answering tens of phone calls in quick succession, replying to even more emails. Despite sitting all day in my small flat in Oxford, I felt part of something meaningful – a small cog in a big machine that was helping to fight COVID-19.
This sense of working together for a common goal was one of the most powerful aspects of the trial. Everyone – doctors, nurses, pharmacists, data analysts and patients – was made to feel part of it, wherever they were. Suddenly regular clinical staff, many of whom had never been given the opportunity to do research before, were part of a major clinical trial that would soon change the trajectory of the COVID pandemic.
In total, around 10% of all those admitted to hospital with COVID-19 in the UK participated in the Recovery trial over the first year. In the city of Leicester alone, well over 1,000 people had taken part by the end of 2020. By itself, this one NHS Trust randomised more patients than most of the world’s largest COVID trials.
Due to its integrated nature, the NHS provided the perfect setting for Recovery. Unlike other healthcare systems in the US and Europe, the NHS is a unified, single-payer system that does not rely on medical insurance. This means in each UK nation, primary care practices and hospital sites all work in collaboration under a common leadership structure.

Drug supplies are provided and managed nationally. Each patient has a unique identifier (NHS number) assigned to them, enabling the linkage of data recorded in different places. Most information used for hospital reimbursement, disease surveillance and clinical auditing is collected centrally for the entire nation.
These features may not have been thought up or designed to enable clinical research but serendipitously, they ended up providing the precise infrastructure that was needed to deploy large-scale randomised COVID trials at rapid pace.
The process for obtaining ethical approval of studies in the NHS is also governed by a central body, the Health Research Authority, which eliminates the need for lengthy and cumbersome approval processes at each participating institution. This could have been a major limitation for Recovery, given that it spanned more than 170 hospitals across the UK. Instead, the study was approved and regularly reviewed by a single research ethics committee, greatly streamlining the entire process.
Throughout the second half of 2020 and beyond, the desire of doctors to help with the wider medical effort against COVID-19 could, in part, be channelled into making the Recovery trial a success. Instead of every department in each UK hospital trying to work out how to best care for their COVID patients, Recovery offered the prospect of a united response: “We don’t know the answers yet, but we do know how to find out – and we’re going to do it together.”
Three groundbreaking results in 100 days
Guilherme – Out of five main COVID-19 treatments recommended for people in hospital by the current NHS guidelines, four were proven effective by Recovery. This gives me an enormous sense of pride whenever I look after someone with COVID in hospital, or hear colleagues discussing their patients. Prescribing one of these treatments comes with a feeling of joy because we helped move medicine forward. That will be our legacy for future generations.
In the first 100 days alone, Recovery produced three groundbreaking results that, almost overnight, changed COVID care around the world.
Early in the pandemic in the US, the antimalarial drug hydroxychloroquine had received emergency-use authorisation by the US Food and Drug Administration (FDA). Around the world, it was being widely touted by highly influential figures including the US president, Donald Trump, and Brazil’s Jair Bolsonaro.
But on June 5 2020, Recovery released its first result, showing the drug had no clinical benefits and hinting at the potential risk of harm. Shortly after this result was made public, the FDA revoked its recommendation. This led to an immediate impact on clinical care strategies around the world.
Owing to the extreme urgency of the situation, Recovery’s approach was to issue a press release as soon as each trial’s findings had been ratified. In every case, this was followed as quickly as possible – usually within a few days – by an open-access “pre-print” scientific paper. The fully peer-reviewed version would follow in due course.
Then on June 16 came Recovery’s landmark dexamethasone result, which concluded: “One death would be prevented by treatment [with dexamethasone] of around eight ventilated COVID-19 patients, or around 25 patients requiring oxygen alone.” This cheap steroid, widely available since the beginning of the pandemic, was now a key element of COVID treatment strategies.
In modern medicine most treatments, such as statins for cholesterol and anti-inflammatory drugs for rheumatic diseases, only have “small-to-moderate” effect sizes – around 10-20% reductions in the likelihood of having a bad outcome. This is comparable to the overall effect seen in Recovery with dexamethasone, although the impact in the sickest patients was larger. While effects of this size may not seem much, when used at the scale of a pandemic or in combination with other treatments with similar “modest” effects, they can have a tremendous impact on population health.
À lire aussi : Dexamethasone: what is the breakthrough treatment for COVID-19?
However, small trials (involving hundreds of people) are not usually sufficient to convincingly identify these beneficial treatments because of the natural differences between people. Recovery was designed to overcome this problem – each possible treatment was studied in many thousands of patients to ensure the results could be relied on, and were not just down to the play of chance.
To conclude a rollercoaster month, Recovery announced its preliminary results on the antiviral drug combination lopinavir/ritonavir in late June 2020. Like hydroxychloroquine, this drug had already been recommended by some national clinical guidelines. It was being widely used around the world based on hints of efficacy in laboratory tests, but had not yet been proven to help hospitalised COVID patients. Recovery showed the drug combination was not effective at preventing deaths in hospitalised patients, helping to redirect busy clinical staff and stretched healthcare resources towards treatments that would actually make a difference.
Mark – After the first wave of the pandemic subsided, an opportunity arose to join Recovery’s central coordinating office as a research clinician. Part of my role was monitoring and extracting evidence about tocilizumab (an immunosuppressing rheumatoid arthritis drug treatment) for a separate meta-analysis alongside the Recovery trial results. By the time those results were imminent in early 2021, my wife was nine months pregnant.
Living through our pregnancy during the pandemic was another reminder of how skilled and dedicated our NHS staff are. Despite everything that was going on, every single person we came across performed their role with compassion and expertise, normalising our experience as best they could. At five o’clock one morning it became clear the contractions weren’t going away. We were blessed with a healthy new son, born at home. By the time I returned from paternity leave, we also knew that tocilizumab saved lives in hospitalised COVID patients, and we never looked back.

A disappointing but equally important result
Leon – I started working with the Recovery coordinating team in late October 2020, mainly on a new treatment that had been introduced to the trial in May. The story of convalescent plasma therapy in COVID-19 illustrates what has made Recovery a success, and why more big trials like it are needed in future.
Convalescent plasma has been tried as a treatment for various infections for more than a century, without any definitive evidence to show whether it works or not. The idea is simple and appealing: people recently recovered from an infection usually have high levels of antibodies in their blood against the pathogen responsible. By collecting that blood and separating the plasma (the antibody-containing liquid in which blood cells are suspended), antibodies can then be given to other people in the early stages of the same infection, in whom it might prevent severe disease or death.
This was one of the earliest treatments tried for COVID-19, but could Recovery find definitive proof of whether it worked?
The most influential early data came from a large US observational study – a non-randomised study comparing people who happened to receive different treatments as part of their medical care. This found that hospitalised COVID patients who received convalescent plasma with high antibody levels had a third lower chance of death than those given plasma with low antibody levels (the control group in this study). This finding was used to justify widespread use of convalescent plasma in the US, where it has been given to hundreds of thousands of COVID patients.
However, this study had serious limitations – not only was there no control group that didn’t receive any convalescent plasma, but the study was not randomised. Many factors determine which treatments different patients receive, including considerations that aren’t reflected in the medical records. For example, we know that experienced doctors often place more weight on how unwell a patient looks than individual test results. If patients receiving a particular treatment are on average sicker than those who don’t, any conclusions about the effects of that treatment could be false.
À lire aussi : Coronavirus: what is plasma therapy?
Non-randomised observational studies can’t typically find a way around this issue, so while they have many uses, they don’t reliably tell us if a particular treatment works. Yet this long-recognised limitation did not stop some misleading observational results being widely promoted and acted upon for the treatment of COVID-19.
What was needed was evidence from a study that was both randomised and sufficiently large to provide clear results. By the time Recovery had finished testing convalescent plasma in January 2021, 10 small randomised trials of the same treatment had already been reported, totalling more than 1,600 COVID patients. Yet even taken together, they were too small to tell us whether convalescent plasma reduced the risk of death by 40%, or had no effect at all. Recovery randomised 11,000 patients, increasing the statistical power more than tenfold, and showed very clearly that convalescent plasma was of no material benefit for patients with severe COVID-19.
While disappointing, this negative result was definitive enough to enable UK research and clinical practice to move on to new COVID treatment targets. In fact, Recovery subsequently showed that higher doses of human antibodies targeted specifically at the virus, but produced in the lab instead of collected from recovered patients, did reduce the risk of death in patients with low antibody levels. Other studies continue to investigate whether convalescent plasma could be effective if given at much higher doses, or very early in an individual’s COVID infection.
We now know more about COVID-19 than most older diseases
Guilherme – Even now, the initial excitement of witnessing practice-changing results still lingers. Medicine is hard, and clinicians need to be familiar with many different treatments and the circumstances in which they are, or are not, likely to be effective. But it is rare for us to know exactly how and when these treatments came to be part of our arsenal – and to feel an emotional connection with those moments.
In all, Recovery has now demonstrated the effectiveness of four life-saving medical treatments for COVID-19, and given clear conclusions on six other treatments that do not work. The effective treatments, which had mostly inconclusive results in other trials, were each found to reduce the risk of death among hospitalised COVID patients by 10-20%. Since their effects are additive, given in combination they can reduce the risk of death by around 40-50% for severely ill patients, compared with the likelihood of death when the pandemic was declared in March 2020.
Because of Recovery, we now know more about the treatment of COVID-19 than most much older diseases. Recovery was part of an extraordinary response by the NHS, the National Institute for Health and Care Research, UK Research and Innovation and many other organisations that, hopefully, won’t need to be repeated soon. However, it offers a roadmap for how much progress could be made in other areas of medicine by randomising more patients in treatment trials, and integrating these trials within routine care to minimise the burden on clinical staff.
Importantly, establishing simple trials with broad eligibility criteria in all UK hospitals opened up Recovery’s research to COVID patients from disparate backgrounds, avoiding the usual under-representations. For example, the ethnic group breakdown in the dexamethasone result is very close to the overall distribution of the UK population, and also the proportions among hospitalised COVID patients. This has helped to provide practice-changing results for all patients both within and outside the UK.
Recovery has always focused on the effect of different drugs on mortality. This ensures that everyone can easily understand the answers the trial delivers: does the treatment save lives or not? Information on mortality can also be easily collected from national databases, providing additional sources of comparison for evaluating longer-term survival.
Recovery was thus able to deliver the sort of certainty that could be understood by everyone – doctors, patients and politicians alike – anywhere in the world, and be put into practice quickly.

What could Recovery mean for the future of drug testing?
Mark – My experience of the pandemic and Recovery has impassioned me to pursue opportunities to narrow the gap between randomised trials and normal NHS clinical care. In my field of infectious diseases, randomised trials of common conditions are barely performed, despite a lack of high-quality evidence in many areas.
Guilherme – Societies cannot flourish when they are not healthy. This was made critically clear by the pandemic. For me, this means working to develop the processes needed to integrate NHS data into randomised trials even further, so we can accelerate and facilitate the delivery of new treatments to patients suffering from diseases other than COVID-19 through a combination of clinical knowledge and large-scale data science. In this way, we can perpetuate the core lessons learnt in the Recovery trial.
The results of the Recovery baricitinib comparison in March 2022 demonstrated that, while some may try to “move on” from COVID-19, the search for new and better treatments continues. Baricitinib (another anti-inflammatory drug used to treat rheumatoid arthritis) was found to reduce death by an additional 13% on top of the mortality reductions achieved with existing treatments. Recovery was the only baricitinib trial that included large numbers of patients receiving other immunosuppressive drugs, meaning we now have more confidence about using combinations of COVID treatments in larger groups of people.
In our view, Recovery could be the catalyst for a seismic shift in the way clinical research is conducted in the UK and elsewhere. The trial has shown that, in a time of crisis, an entire country can break through the established status quo to collaborate together for a common goal, to enormous effect.

Tens of thousands of patients are admitted to hospital each year for conditions such as heart attacks, influenza, stroke and pneumonia. These diseases kill large numbers of people each year – many more than COVID-19 – yet the evidence base for treatment of some of them is now of worse quality.
Enrolling the majority of these patients into nationwide, randomised trials such as Recovery could provide definitive answers to many treatment dilemmas within months. Decisions about which treatments are better for a particular patient group are being made by doctors every day, based on limited evidence and without improving the evidence base for future patients. Instead, through a national randomised trial programme, we could all be learning with each patient who requires clinical care.
Furthermore, such opportunities for clinical research should not be limited to the UK. Recovery is now recruiting patients in six countries in Africa and Asia. By including clinically relevant common diseases and evaluating widely-available treatments, future benefits could be accessible across the world. Large collaborative trials can be managed via online resources, randomisation can be performed online, and (where available) follow-up can be facilitated through electronic healthcare records and simple questionnaires.
We also hope that governments and research funders can make it easier for similar large trials to happen – and for doctors and patients everywhere to be involved. The ball has already started rolling at the highest levels including the G7, but this transformation needs continued support.
À lire aussi : Testing sewage has helped track COVID – soon it could reveal much more about the UK's health
Recovery has shown that these trials do not require complicated technology, expensive drugs or fancy laboratories. In the UK, pragmatic trials can be run at exceedingly low cost by making better use of the wealth of existing NHS clinical research and healthcare data infrastructure. Using this approach, we could achieve small but continuous improvements in healthcare that, when combined, lead to increased longevity and reduced disability, easing the strain on our health services. This would represent a major financial saving for the UK as a whole.
Recovery has also underlined the importance of embedding clinical trials in routine medical practice. Well-designed trials are carried out with patients and for patients in a healthcare system that recognises we don’t always know which treatments work, but that is committed to finding out. Through such a system we can achieve multiple, incremental improvements in patients’ health outcomes, and focus scarce medical resources on those things we know work while abandoning the many that do not.
As a result of Recovery, the chances of survival for a patient admitted to hospital with COVID-19 today are substantially better than they were two years ago. Now is the time to apply these lessons to the many other health challenges we face.
This is the first in a series of Insights articles developed with UK Research and Innovation (UKRI) to explore the wider impacts and implications of COVID-19 research

For you: more from our Insights series:
To hear about new Insights articles, join the hundreds of thousands of people who value The Conversation’s evidence-based news. Subscribe to our newsletter.
Les auteurs ne travaillent pas, ne conseillent pas, ne possèdent pas de parts, ne reçoivent pas de fonds d'une organisation qui pourrait tirer profit de cet article, et n'ont déclaré aucune autre affiliation que leur organisme de recherche.
This article was originally published on The Conversation. Read the original article.







