READ THE FULL SQZ RESEARCH REPORT
ESMO-IO Preliminary Results and Webcast/Conference Call
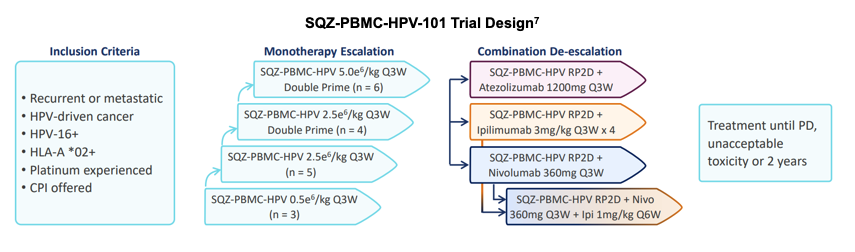
SQZ Biotechnologies Company (NYSE:SQZ) hosted a webcast and conference call on December 9, 2021 to discuss updates to SQZ-PBMC-HPV-101 program at ESMO1 Immuno-Oncology Congress 2021. SQZ-PBMC-HPV-101 is the company's antigen presenting cell (APC) lead program. Jong Chul Park, MD presented, "Preliminary results from the first-in-human, dose-escalation study of a cell-based vaccine in HLA A*02+ patients with recurrent, locally advanced, or metastatic HPV16+ solid tumors." In contrast to cross-presentation, the basis for traditional prophylactic vaccines, Cell Squeeze is able to elicit a thousand-fold increase of T Cell activation. Cross presentation favors creation of CD4+ helper cells and antibodies while Cell Squeeze prioritizes creation of CD8+ T cells via a different presentation mechanism of squeezing vs endocytosis.
In previous releases Cell Squeeze had reported vein-to-vein time of about one week with a median manufacturing time of just 17 hours, a testament to the quick turnaround time of Cell Squeeze technology can achieve. In the latest study, not only were Cell Squeeze doses produced efficiently, but also with high yield and viability.
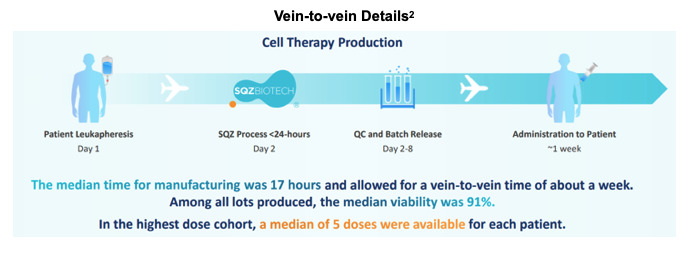
Out of the 18 patients generating results, the only serious adverse event (AE) reported was one case of grade 2 cytokine release syndrome (CRS) in a first cohort patient. The event resolved in less than 24 hours. There was one case of grade 3 anemia in a second cohort patient and no patients met dose limiting threshold criteria.
The presentation highlighted one patient in particular, Patient 17, who showed noteworthy response and was featured as a case study in the presentation. The patient was a 52 year old man with oropharynx squamous cell carcinoma: a large primary lesion with significant burden from symptoms.
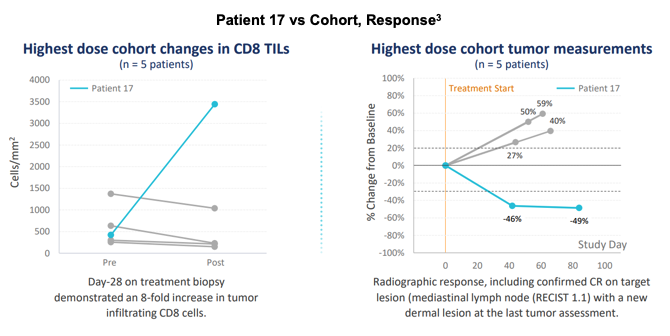
Patient 17 showed outsized presence of CD8+ tumor infiltrating lymphocytes (TILs) versus other members of the highest dose cohort. Similarly, Patient 17 demonstrated a marked decrease in the tumor as evidenced by radiography, including confirmed Complete Response on target lesion. Patient 17 was diagnosed 3.7 years prior to SQZ therapy with six prior lines of systemic therapy including carboplatin/Fluorouracil (5FU)/pembrolizumab and anti-TGFβ/pembrolizumab (best overall response of progression of disease) nine months before SQZ-PBMC-HPV dosing. He received all seven doses of SQZ-PBMC-HPV with excellent tolerability. Dysphagia and neck swelling improved and physical examination of the lesion also improved.
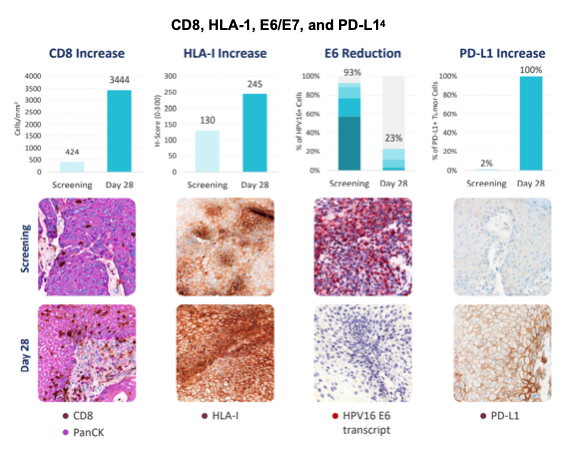
In addition to the enhanced CD8+ presence within the tumor, which is associated with positive prognostic value,5 Patient 17 demonstrated increase in HLA-1, reduction in E6/E7 (target antigen) and increase in PD-L1 which suggest potential synergy with a combination approach. PD-L1 acts as a brake, tempering the body's immune response, which is undesirable in this therapeutic application. Thus, use of SQZ-PBMC-HPV in combination with a PD-L1 blockade agent could augment therapeutic efficacy even further.
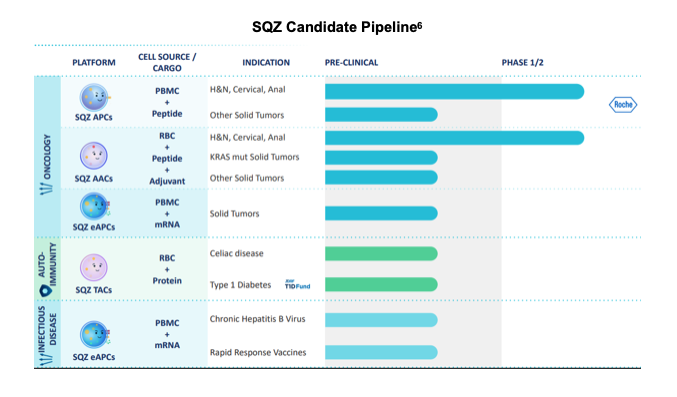
SQZ is pursuing HPV+ cancer based on the unmet need in a global disease. HPV+ cancers are also well-studied, providing standardized markers of success for the company's work. HPV+ tumors in patients that are HLA-A*02+ have been extensively researched and there are many known biomarkers that can be leveraged to assess therapeutic activity and monitor patient metrics. Demonstrating efficacy in HPV serves as excellent proof of concept for this novel technology and has applications in multiple solid tumor types. SQZ' candidate is being evaluated in a Phase I study and has licensed certain commercialization rights to Roche. The cells are engineered, autologous peripheral blood mononuclear cells (PBMCs) squeezed with HPV+ solid tumor specific antigens. Squeezing, in contrast to other delivery methods, enables robust MHC-I presentation of the target and drives activation of the patient's endogenous CD8+ T cells against the HPV+ tumor cells.
Operational Highlights Year-to-Date:
➢ IND cleared for SQZ AACs in HPV+ tumors - February 2021
➢ Announcement and pricing of $56.4 million offer - February 2021
➢ 1Q:21 financial results and recent business highlights - May 2021
➢ B. Coulie, MD, PhD and P. Vink, MD appointed to Board, Moessinger exits - July 2021
➢ 2Q:21 financial results and portfolio updates - August 2021
➢ 3Q:21 financial results and portfolio updates - November 2021
Development Milestones
➢ APC HPV IND cleared - October 2019
➢ APC HPV Phase I launched - January 2020 (SQZ-PBMC-HPV)
➢ APC HPV ASCO interim data presentation - June 2021
➢ AAC HPV IND cleared - January 2021
➢ AAC HPV Phase I initiated - August 2021
➢ DSMB8 recommends trial advance to CPI9 combo stage - October 2021
➢ Acceptance by Roche Accelerator in China - December 2021
➢ Enhanced APC IND filing - 4Q:21
➢ Enhanced APC trial launch - 1H:22
➢ AAC HPV data presentation - 2022
➢ Enhanced APC data presentation - 2H:22
➢ IND for Immune Tolerance in celiac disease - 3Q:22
➢ eAPC IND filing in HBV - 2H:22
➢ AAC or eAPC IND for KRAS mutant solid tumors - 2H:22
Presentations:
➢ SQZ tolerizing antigen carrier platform preclinical data presented at ASIT10 Digital Summit - January 2021
➢ Presented preclinical data on mRNA eAPCs in KRAS tumors at AACR 2021 - April 2021
➢ New data on rapid cell therapy manufacturing at ASGCT 2021 - April 2021
➢ Preliminary results for SQZ-APC Phase I HPV16+ tumors at ASCO11 2021 annual meeting - June 2021
➢ Preclinical data for TACs in autoimmune type 1 diabetes at FOCIS12 annual meeting - June 2021
➢ iPSC neuron generation with mRNA transcription factor at 2021 ISSCR13 - June 2021
➢ Poster presentations for three oncology programs at SITC14 - November 2021
➢ Highest dose monotherapy cohort oral presentation at ESMO-IO15 - December 2021
Summary
During the recent webcast, SQZ updated investors on its current trial investigating SQZ-PBMC-HPV in HPV16+ tumors. The candidate was found to be safe and well tolerated across all dose levels. Product batches were produced under cGMP yielding multiple doses within less than 24 hours, for a total vein-to-vein time of about one week. Radiographic data correlated with patient clinical benefit. In particular, Patient 17 demonstrated clinical response that was in line with the expected SQZ APC mechanism. The patient had increased CD8+ tumor infiltration, reduction of cells expressing E6 and E7 antigens, and increased PD-L1 expression. The 5.0M cells/kg dose cohort was given the nod by the Data Safety and Monitoring Board to transition into PD-L1 and CTLA-4 combination cohorts, while high dose monotherapy SQZ-PBMC-HPV continues enrollment to further characterize efficacy. SQZ' portfolio offers treatments for multiple indications using a simple approach to engage the body's immune response. Results from SQZ' lead program provide further evidence that the APC platform initiates an immune response in patients that have failed a number of other lines of therapy.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.
________________________
1. European Society for Medical Oncology
2. PowerPoint Presentation (q4cdn.com)
3. PowerPoint Presentation (q4cdn.com)
4. PowerPoint Presentation (q4cdn.com)
5. Bruni et al, Nature Reviews 2020
6. SQZ Investor Deck November 2021
7. PowerPoint Presentation (q4cdn.com)
8. Data and Safety Monitoring Board for Phase I/II clinical trial SQZ-PBMC-HPV-101
9. Checkpoint inhibitors, PD-L1 and CTLA-4
10. Antigen-Specific Immune Tolerance
11. American Society of Clinical Oncology
12. Federation of Clinical Immunology Sciences
13. International Society for Stem Cell Research
14. Society for Immunotherapy of Cancer
15. European Society for Medical Oncology Immuno-Oncology







