Humanity could establish bases on other planets and guarantee their supply of oxygen using new techniques discovered here on Earth.
The discovery — Research published earlier this month reveals that the water electrolysis process, which uses electricity to split the water particles into hydrogen and oxygen, does indeed work at lower gravity levels. The findings, published in the journal Nature, show that oxygen production via electrolysis only reduced by 11 percent under Moon-like gravity conditions.
“If there is water-ice on Mars, then this could also be electrolyzed to make oxygen and hydrogen,” Mark Symes, a senior lecturer in chemistry at the University of Glasgow and an author on the paper, tells Inverse.
“These could then be used to propel rockets from Mars back to Earth.”
Why it matters — The findings could help enable some of the most ambitious plans in spaceflight. In 2017, SpaceX CEO Elon Musk outlined plans to send humans to Mars in the 2020s, establish a base, and ultimately establish a city by 2050.
The Starship, an under-development reusable rocket, uses liquid oxygen and methane as its fuel. At the 2017 event, Musk explained how astronauts could collect water and carbon dioxide and use it to make fuel:
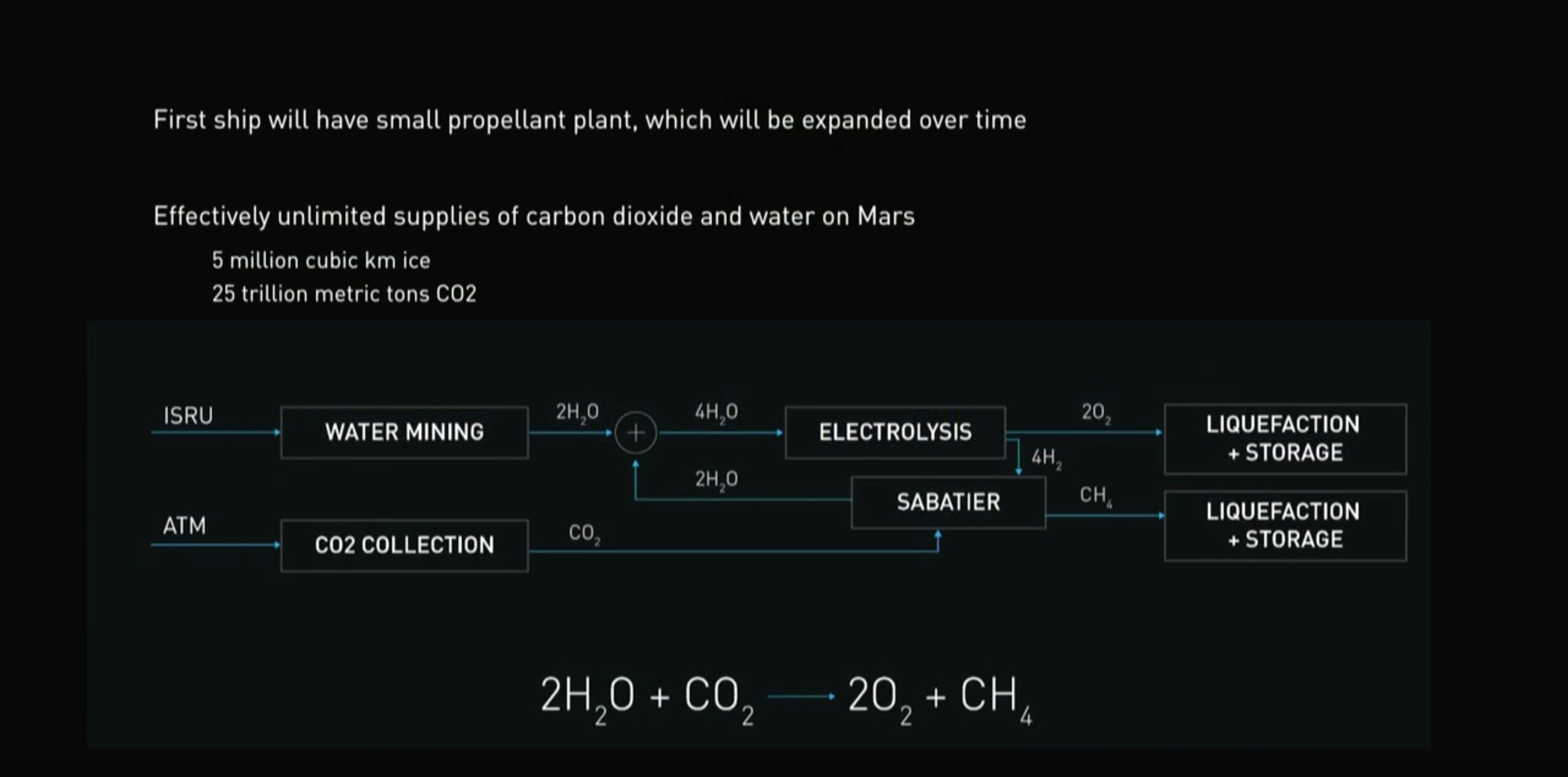
As the diagram above shows, electrolysis is vital for making the liquid oxygen that ships could use to return.
How they did it — The latest research looked at how reduced gravity could change the electrolysis process. The team conducted electrolysis at several strengths of gravity, ranging from 0.166 g (similar to the Moon) to 8 g (eight times stronger than Earth). By comparison, Mars’ gravity is around 0.38 g.
“No one had previously looked at the effects of lunar gravity on the water electrolysis process,” Symes says.
The team found that oxygen production was only reduced by 11 percent under the weakest, Moon-like gravity. Most importantly, the process works and could provide resources for a lunar base.
What this means for humans on the Moon
NASA has already started experimenting with using Mars’ resources. In April 2021, the agency’s Mars Oxygen In-Situ Resource Utilization Experiment, or MOXIE, extracted its first oxygen from the air. It did this by collecting carbon dioxide from the atmosphere and using a different electrolysis process.
MOXIE was a success, but a future version would need to be much bigger. The current version, the size of a toaster, produces 10 grams of oxygen per hour. A four-person astronaut mission would need around 55,000 pounds of oxygen to leave Mars, which would require a MOXIE around 100 times larger.
With these latest findings, astronauts have an alternative source of oxygen. Explorers could seek out water-ice and use that to harvest more oxygen.
Looking to the far future, Musk and others want to build large cities and even terraform the planet to make it more liveable. What do the latest findings mean for that?
“That’s a bit beyond my expertise, but in general I would say that anything that allows or facilitates humans spending time on these other planets without constant resupply from Earth is a step towards a self-sufficient infrastructure,” Symes says.
Abstract: Establishing a permanent human presence on the Moon or Mars requires a secure supply of oxygen for life support and refueling. The electrolysis of water has attracted significant attention in this regard as water-ice may exist on both the Moon and Mars. However, to date there has been no study examining how the lower gravitational fields on the Moon and Mars might affect gas-evolving electrolysis when compared to terrestrial conditions. Herein we provide experimental data on the effects of gravitational fields on water electrolysis from 0.166 g (lunar gravity) to 8 g (eight times the Earth’s gravity) and show that electrolytic oxygen production is reduced by around 11% under lunar gravity with our system compared to operation at 1 g. Moreover, our results indicate that electrolytic data collected using less resource-intensive ground-based experiments at elevated gravity (>1 g) may be extrapolated to gravitational levels below 1 g.