
What do plants do during a solar eclipse?
As the total solar eclipse crossed South America, it wasn’t just people oohing and ahhing as the sun was blotted out. Other living things had their own responses, too – some of which we are just beginning to understand. As some scientists used the Great American Eclipse of August 2017 to watch how bees and birds dealt with sudden midday darkness, researchers in Wyoming investigated big sagebrush, a shaggy, aromatic desert shrub that grows throughout the western United States.
Tracking its reactions at the leaf level, scientists saw it experience a slowdown in activity as darkness fell, followed by shock at the sun’s surprise return. The study, published in Scientific Reports, adds to a small clump of botanical eclipse research, all produced by people with the ability to wonder, even as a celestial event occurs: What are the plants getting up to?
“Plants haven’t really been well-documented during the eclipse,” says Daniel Beverly, a doctoral student in botany and hydrology at the University of Wyoming and the paper’s lead author. When he heard an eclipse was coming, he saw the chance for “a once-in-a-lifetime data set”.
Big sagebrush spends much of its life in the sun and “it covers such a large portion of the country”, from Oregon down to New Mexico, Beverly says, making it a good subject for study. He and his colleagues chose a patch close to Yellowstone National Park. They set up instruments that could measure photosynthetic rate, as well as the speed of transpiration – how quickly its leaves lose water. As the light faded and the temperature dropped during the 80 or so minutes before totality, Beverly and his team saw a corresponding decrease in photosynthesis and transpiration. “The plant responded as if it was dusk,” he says.
During the two minutes and 18 seconds of complete darkness, both rates fell further. Although they did not quite reach the slowdown level of an average nighttime, it was a much more precipitous drop than would have occurred for a passing cloud. But when the sun appeared again, the shrubs were blindsided. Over the course of the eclipse day, the team estimated, your average big sagebrush managed about 14 per cent less photosynthesis than it would have if the sun hadn’t been blocked. If a plant is already drought-stressed, an eclipse might be bad news, like “losing 14 per cent of a day’s income when you’re already broke”, Beverly says.
Scientists took an MRI scan of an atom
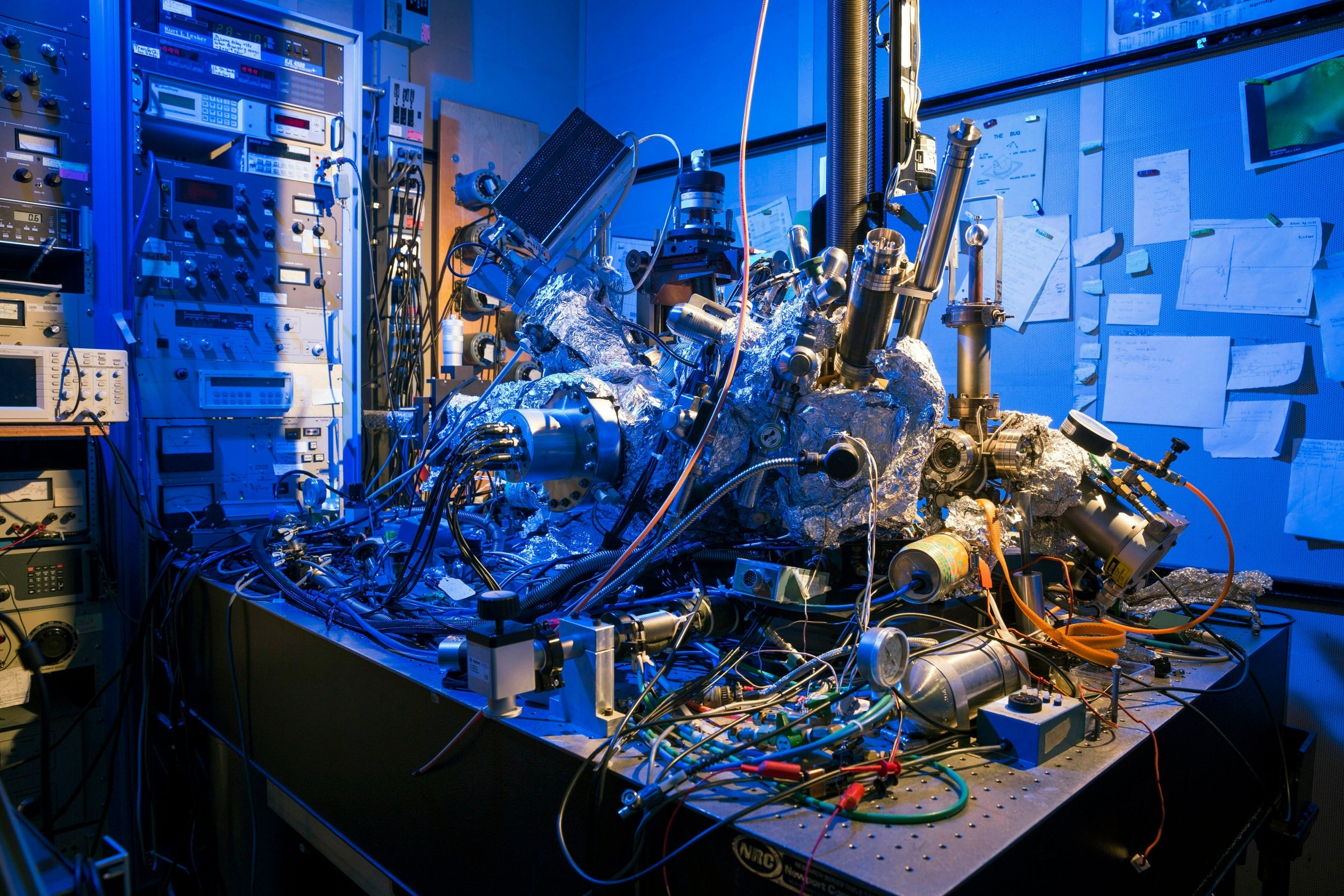
As our devices get smaller and more sophisticated, so do the materials we use to make them. That means we have to get up close to engineer new materials. Really close. Different microscopy techniques allow scientists to see the nucleotide-by-nucleotide genetic sequences in cells down to the resolution of a couple of atoms as seen in an atomic force microscopy image. But scientists at the IBM Almaden Research Centre in San Jose, California, and the Institute for Basic Sciences in Seoul have taken imaging a step further, developing a new magnetic resonance imaging technique that provides unprecedented detail, right down to the individual atoms of a sample.
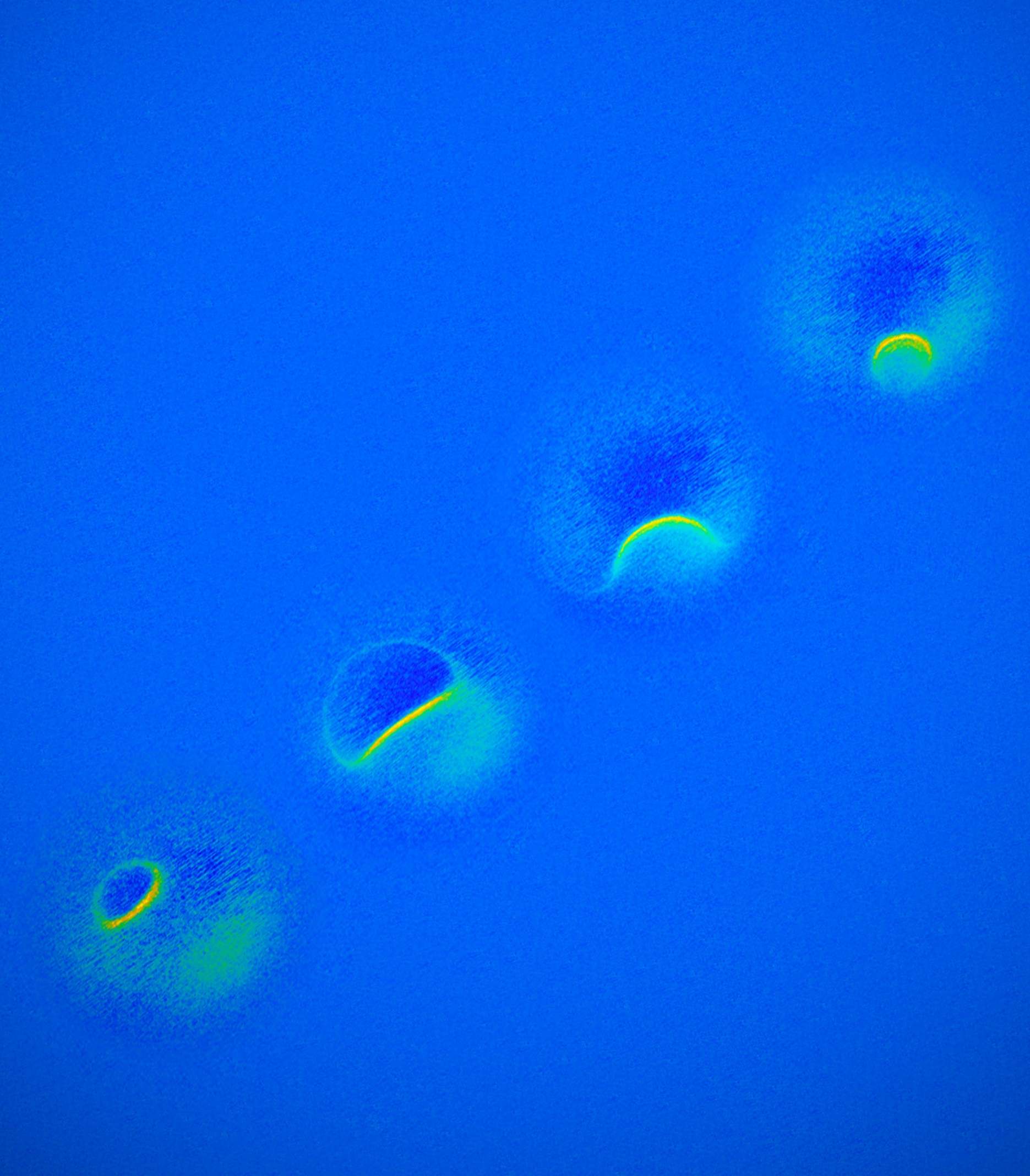
The technique relies on the same basic physics behind the MRI scans that are done in hospitals. When doctors want to detect tumours, measure brain function or visualise the structure of joints, they employ huge MRI machines, which apply a magnetic field across the human body. This temporarily disrupts the protons spinning in the nucleus of every atom in every cell. A subsequent, brief pulse of radio-frequency energy causes the protons to spin perpendicular to the pulse. Afterwards, the protons return to their normal state, releasing energy that can be measured by sensors and made into an image.
But to gather enough diagnostic data, traditional hospital MRIs must scan billions and billions of protons in a person’s body, says Christopher Lutz, a physicist at IBM. So he and his colleagues decided to pack the power of an MRI machine into the tip of another specialised instrument known as a scanning tunnelling microscope to see if they could image individual atoms. The tip of a scanning tunnelling microscope is just a few atoms wide. And it moves along the surface of a sample, it picks up details about the size and conformation of molecules.
The researchers attached magnetised iron atoms to the tip, effectively combining scanning-tunnelling microscope and MRI technologies. When the magnetised tip swept over a metal wafer of iron and titanium, it applied a magnetic field to the sample, disrupting the electrons (rather than the protons, as a typical MRI would) within each atom. Then the researchers quickly turned a radio-frequency pulse on and off, so that the electrons would emit energy that could be visualised. The results were described in the journal Nature Physics.
“It’s a really magnificent combination of imaging technologies,” says A Duke Shereen, director of the MRI Core Facility at the Advanced Science Research Centre in New York. “Medical MRIs can do great characterisation of samples, but not at this small scale.”
Drugged, castrated, eager to mate: the lives of fungi-infected cicadas

Beneath the soil, cicadas wait – as long as 17 years – until the right temperature beckons them to the surface. At once, they emerge, like zombies rising from graves. They greet the sun, preparing to moult into adults in the coming weeks and then fly off to mate. Such is a cicada’s simple life – unless it’s been infected.
Massospora, a parasitic fungus, has lurked just below the surface, awaiting the cicada’s exit. When a nymph digs through infected soil, fungal spores cling to its body. As the cicada matures, massospora multiplies, digesting the insect’s insides, castrating it and replacing its rear end with a chalky white plug of spores. The cicada buzzes on, seemingly unaware it’s a mushroom’s moving minion. It flies, attempting to mate with unusual vigour. Some males even mimic females to attract and spread their spores to male partners – the more infected, the better. And as their hijacked bodies copulate, spores sprinkle to the earth and massospora spreads.
“This really has all the elements of a sci-fi horror story,” Matthew Kasson, a mycologist at West Virginia University, says. “How does an organism function even though two-thirds of their body is no longer theirs?”
Perhaps, he thinks, the fungus dopes it with psychoactive drugs.
As reported last year in a paper that had not yet been through peer review and in The Atlantic, Kasson and colleagues discovered the infected cicadas’ plugs were laced with an amphetamine called cathinone, found only in the khat plant of the Horn of Africa and the Arabian Peninsula, and psilocybin, the hallucinogen that makes magic mushrooms magic. At the time, his team assumed massospora synthesised these drugs on its own, following the same recipes in khat and magic mushrooms. But a follow-up genomic analysis, published recently in Fungal Ecology, revealed massospora didn’t have the right ingredients. Its drug lab had evolved independently. It also shows that for prospectors seeking pharmaceutical advances in nature, there are more drugs to be found in unexpected places.
When they sequenced the genomes of massospora fungi species that infect cicadas, they expected to find the genes used by magic mushrooms or khat to produce the narcotic substances. But those genes were missing. One possible explanation is that the fungus and cicada, maybe including the microbes living in its gut, co-evolved a unique interaction to produce these substances. But what would the cicada get out of it?
Kasson can only speculate. Maybe drugs make cicadas less desirable to predators. Maybe they make cicadas fearless. “Maybe the cicadas just want to feel numb,” he says.
Crocodiles went through a vegetarian phase, too
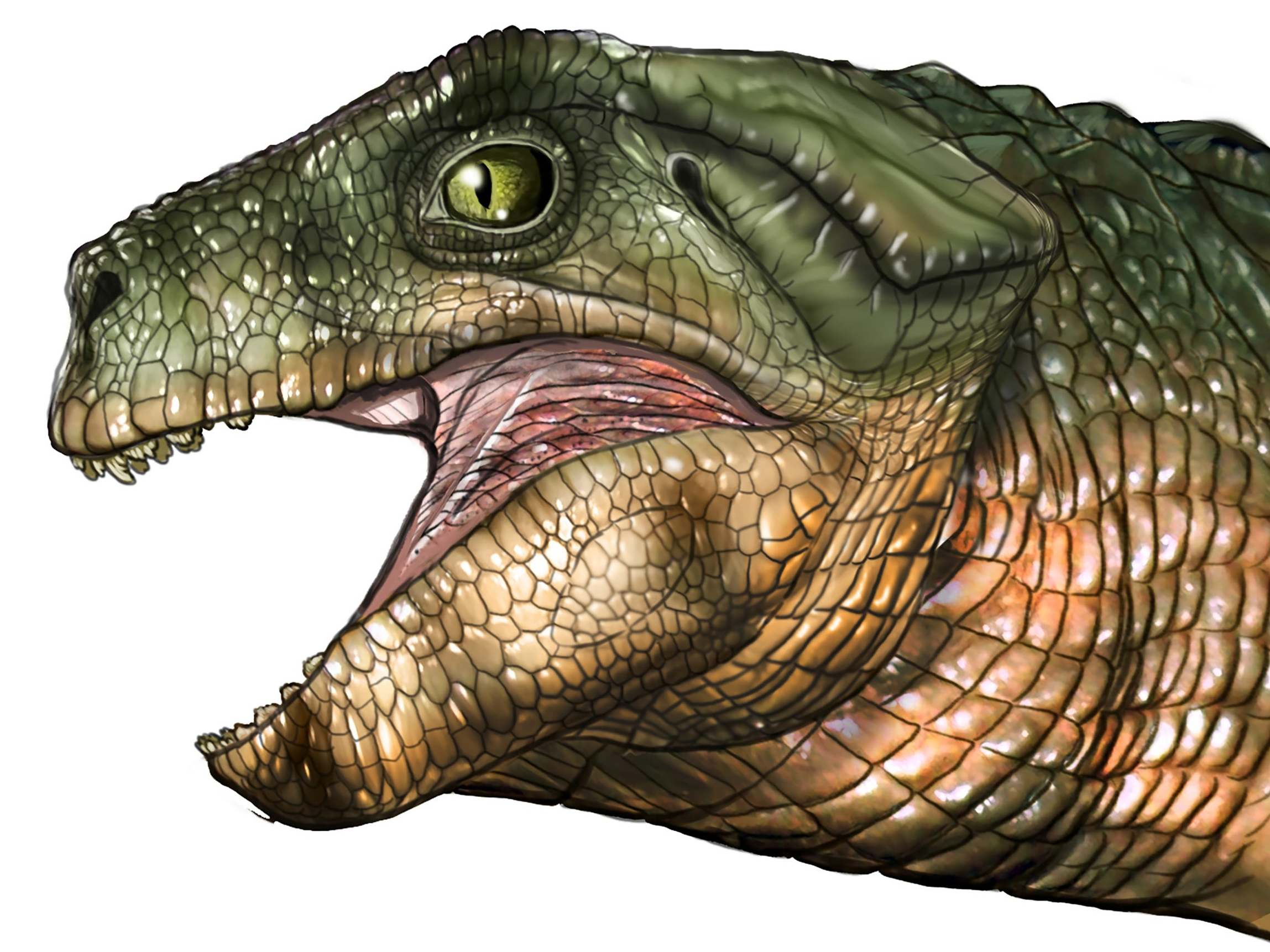
Imagine you’re a small mammal of the Mesozoic. Snuffling around one day, you run into a cat-sized, scaly, big-eyed reptile that looks not unlike a crocodile found later in the 21st century. Spotting you, he opens his mouth wide to reveal... tiny, intricate teeth. Then he turns his head and munches on some leaves. Such encounters may have been common in prehistory. Research published in Current Biology suggests that vegetarianism evolved at least three separate times in ancient crocs – a conclusion reached after scientists studied the unusual teeth sported by many species, including the Simosuchus described above.
Today, crocodiles and their relatives, among them alligators, caimans and gharials, can be found across the southern hemisphere. They have many things in common, including meat-heavy diets, a penchant for swimming and their teeth. Ask them to smile for a family reunion photo, and each mouth would bristle with simple, blunt-tipped cones. But the Mesozoic was a different story. About 250 million years ago, scores of crocodyliform species could be found across the globe, some on land and some in seas and rivers. A particular species might eat only plants, only animals, or both. To support these varied diets, many had “unique, interesting teeth”, says Keegan Melstrom, a geobiology graduate student at the University of Utah and lead author of the new study.
Melstrom has been keen on crocodile teeth since 2011, when he saw a presentation on an extinct crocodyliform called Pakasuchus. Pakasuchus had canines in the front of its mouth and molars in the back. When its jaw closed, the teeth would neatly slot together – more like a mammal’s mouth than the akimbo grin of modern crocodiles. “It just blew my mind,” Melstrom says. For the new study, Melstrom and his co-author, Randall Irmis, analysed 146 teeth from 16 extinct crocodyliform species. They used a method called orientation patch count rotated. From a scan of an object, the method generates a numerical score indicating the complexity of the object’s shape. “It allows us to compare teeth that have no landmarks in common,” says Melstrom.
This proved especially useful for studying prehistoric crocs, he says, whose teeth often have “no modern-day analogues”. The researchers gathered the complexity scores of the teeth and compared them with those of living reptiles and mammals with known diets. Half of the ancient species seemed to have been on the plant-eating end of the spectrum – “a genuine surprise”, Melstrom says.
Out of their eggs, into the sky: how baby pterosaurs may have taken flight

Modern nature teaches us that flying isn’t child’s play. Newborn birds spend their early days in the nest, and bat pups don’t take off for weeks on end, often requiring prodding from their mothers. But what about baby pterosaurs?
Some researchers think the young of these flying reptiles that lived in the time of the dinosaurs also stayed on the ground for a while, tended by adults. Others argue the opposite: young pterosaurs could immediately fend for themselves, hatching and heading straight for the skies. A paper published in Proceedings of the Royal Society B supports this stirring vision, marshalling evidence from all known pterosaur embryos to argue that the prehistoric creatures were flight-ready from birth.
The researchers took advantage of a fossil cache unearthed in northwest China in 2017. Sometime between about 145 and 100 million years ago, a flood rushed through the area and buried an entire colony of pterosaurs, says David Unwin, a palaeobiologist at the University of Leicester and the study’s lead author. The site has “adults and juveniles, and it’s got lots and lots of eggs”. But attempts to figure out the stage of development each embryo had reached have been “kind of ad hoc – just look-at-it-and-guess”, Unwin says. He and Charles Deeming, a zoologist at the University of Lincoln, set out to standardise the process.
The pair used fossils from that site, along with eggs and embryos from Argentina and elsewhere in China. They first looked at limb lengths, along with egg size and shape. The researchers found that in general, smaller, narrower eggs represent early-stage embryos, while larger and rounder ones indicate a later stage. Next, they examined patterns of bone ossification, or hardening, looking at embryos along with young pterosaurs, called flaplings.
Because bones harden in a particular order, they can serve as “developmental markers”, Unwin says. They then matched these patterns to those observed in quails and alligators, both considered modern analogues of pterosaurs. This helped to sort the pterosaur embryos, from newly laid to about to hatch. Along the way, they noticed something about one bone, the manus digit IV. Equivalent to our ring finger, this is a pterosaur’s “wing finger”, the long, flexible appendage that is attached to its wing membrane and allowed the animal to fly. In most vertebrates, that bone is one of the last to harden. Pterosaurs, though, “ossify it very early”, he says.
To Unwin, this – along with similarly early ossification of other important flight-related bones – is further evidence that pterosaurs could get airborne right out of the egg.
© New York Times







