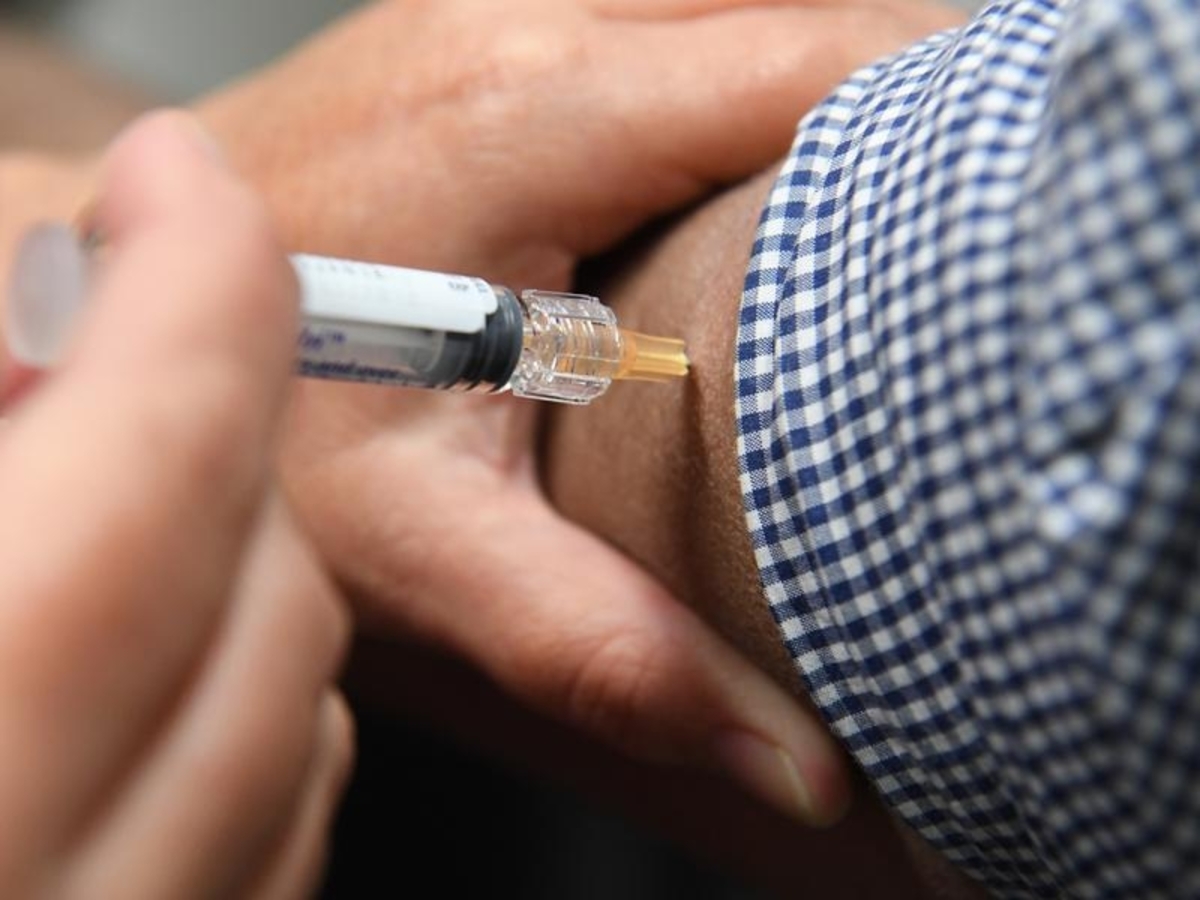
The Moderna coronavirus vaccine is one step closer to being approved in Australia after the medicines regulator gave the green light for an application.
The Therapeutic Goods Administration has granted a provision determination to the company, which the federal government has contracted to provide 25 million doses.
"It is anticipated that Moderna will submit an application for provisional registration shortly," the TGA said in a statement on Thursday.
"Importantly, registration and supply in Australia will only commence should the vaccine be approved as safe and effective by the TGA."
Moderna, like Pfizer, is an mRNA vaccine which teach cells how to make a protein to trigger an immune response.
The RNA from the vaccine does not change or interact with people's DNA in any way.
The federal government has forecast a national distribution of between 87,000 and 125,000 doses a week of Moderna from September.
That is expected to ramp up to between 430,000 to 615,000 a week later in the year.
Moderna has been widely used in countries more advanced than Australia in immunisation including the United States.







