READ THE FULL LEXX RESEARCH REPORT
Since Our Initiation
Since our September 20th initiation on Lexaria Bioscience Corporation (NASDAQ:LEXX), the company has announced several important research and development updates. With DehydraTECH's wide applicability enabling oral administration and numerous active programs, Lexaria is frequently providing updates, especially from animal studies which are seeking proof of concept. DehydraTECH (DHT) has now been granted patents for use with cannabinoids, nicotine, NSAIDs, and vitamins with patents pending for phosphodiesterase inhibitors, estrogen/ testosterone, anti-viral drugs and more. The company has announced an ambitious portfolio of preclinical studies evaluating the effect of CBD on Alzheimer's disease to diabetes and other maladies that make up part of the 12 programs that are planned to be active in 2022.
Highlights for calendar 4Q:21 include:
➢ Oral nicotine study NIC-A21-1 results - October 2021
➢ Oral THC study THC-A21-1 results - October 2021
➢ EPIL-A21-1 commenced - November 2021
➢ HYPER-H21-4 announced - November 2021
➢ 2022 R&D program plans announced - November 2021
➢ Interviews (one, two, three) with Zacks' analyst John Vandermosten - December 2021
➢ HYPER-H21-2 results - December 2021
➢ HYPER-H21-3 nears completion - December 2021
Lexaria preclinical trials planned for 2022:
➢ HOR-A22-1
◦ Estrogen delivery characteristics
◦ Estrogen
◦ PK study
◦ April 2022
➢ DEM-A22-1
◦ Dementia/Alzheimer's disease
◦ July 2022
➢ RHEUM-A22-1
◦ Rheumatoid disease
◦ CBD
◦ October 2022
➢ DIAB-A22-1
◦ Diabetes
◦ CBD
◦ November 2022
HYPER-H21-4
Lexaria announced this autumn that it would evaluate DehydraTECH-CBD in humans in 2022 with its HYPER-H21-4 study. HYPER-H21-4 will target 60 volunteers between the ages of 45-70 using three 150 mg doses of DehydraTECH-CBD every day for the 6-week duration of the study. The study will employ a double blinded, randomized cross-over design and utilize a placebo control. A subset of subjects will already be using standard of care hypertension drugs such as ACE inhibitors and diuretics. This will yield data relating to the efficacy of DehydraTECH-CBD in combination with hypertension treatments. Furthermore, the longer duration of the study compared with Lexaria's previous work will support acquisition of critical data regarding the extended use of DehydraTECH-CBD and capture any potential for longer term health benefits. Study protocols are being readied for submission to the Independent Review Board (IRB) and approval is anticipated by January 2022. HYPER-H21-4 will build on the findings of HYPER-H21-2. Results for HYPER-H21-2 were announced in December 2021 and only tracked the effects of dosing over a 24 hour period yet achieved statistically significant results. Success with HYPER-H21-2 is expected to provide the data necessary to enter regulatory pathways for DHT CBD to treat hypertension and other forms of cardiovascular disease.
HYPER-H21-2 Results
HYPER-H21-2 was an in-human study of DehydraTECH-CBD in arterial stiffness, a strong predictor of many aspects of human disease such as coronary heart disease/hypertension but also diabetes mellitus, renal disease and others. Arterial stiffness can have prognostic value in asymptomatic individuals without overt cardiovascular disease. Arterial stiffness was measured through pulse wave velocity (PWV) evaluation, together with assessments of augmentation index and pressure. All comparisons between DehydraTECH-CBD and placebo were statistically significant (p < 0.01).
PWV is a measure of arterial elasticity and is determined by measuring the speed at which pressure waves move downstream in the blood vessel. Contraction of the left ventricle produces a pulse wave that expands throughout the arterial system. When arterial walls are more pliable, the pulse-driven pressure wave moves more slowly, and when they harden, usually due to age or high blood pressure, wave speed increases. There are reflected pressure waves that return to the source of the pulse at the end of the systolic period which can cause additional work for the cardiovascular system. PWV can be quantified by using two pressure catheters placed at a fixed distance from one another to measure the pulse transit time.
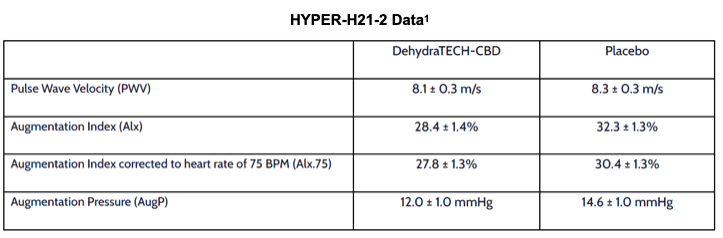
As first reported on September 7, 2021, at times during HYPER-H21-2, some subjects experienced reductions of as much as 20 mmHg in blood pressure relative to placebo. When averaged over the 24-hour duration of the study, reductions in diastolic, systolic and mean arterial pressure were all significantly demonstrated with DehydraTECH-CBD compared to placebo. All secondary objectives of HYPER-H21-2 have now been successfully completed.
On average, PWV values increase by 0.9 m/s every 10 years between the age of 45 and 60 as a function of aging-induced increases in arterial stiffness.2 Sources estimate that a 1.0 m/s increase in PWV accounts for a 15% increase in cardiovascular and all-cause mortality.3
HYPER-H21-2 was conducted at a European medical research hospital. 16 volunteers (8 male, 8 female) aged 45-65 with otherwise untreated pre- or mild-hypertension were given either a placebo, or three separate doses of 150 mg each of DehydraTECH-CBD over a 14-hour period and studied over a 24-hour duration.
THC-A21-1 Results
The most common use for medical cannabis in the US is for the control of pain.4 Chronic pain is associated with nerve disorders and multiple sclerosis and users are frequently cited as responding to a cannabis treatment program, without the highly addictive or sedating effects of opiates. In early work to evaluate the delivery characteristics of DHT, Lexaria conducted its THC-A-21-1 in vivo study of DehydraTECH-THC in Sprague Dawley rats (n=20). On October 13, 2021, it reported that DehydraTECH-THC successfully elevated THC levels in blood plasma, requiring only 15 minutes at levels comparable to those achieved in 45 minutes with concentration-matched controls.
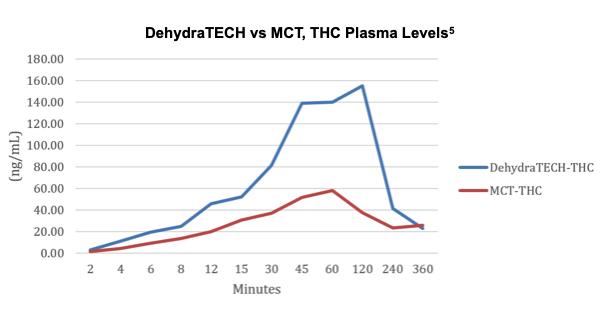
During the study, DehydraTECH-THC outperformed industry standard medium chain triglyceride (MCT/coconut oil) based control formulation from the 2-minute mark onwards, then dropped rapidly to the same level as the MCT control by the 6-hour mark.
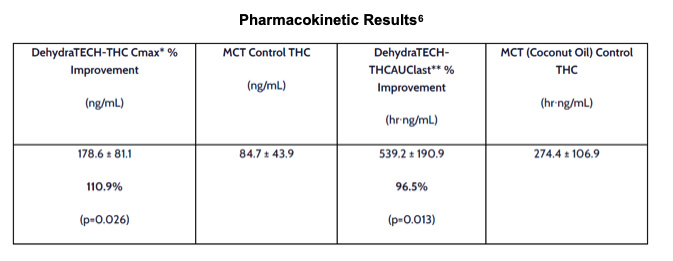
In measures of peak plasma concentration, as well as integration of THC presence in plasma over time, DehydraTECH-THC significantly outperformed MCT control. DehydraTECH-THC was twice as effective as MCT as a facilitator of oral administration of THC.
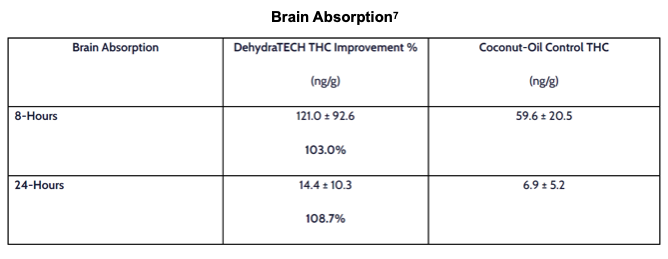
DehydraTECH-THC was not only able to elevate peripheral blood plasma concentrations, but also penetrate into the brain. Data from two time-points were collected. Similar to the peripheral blood data, the brain data indicated effectiveness that was about twice that of the MCT control. The data strongly support further inquiry into DehydraTECH as a facilitator of oral administration of THC.
Study THC-A21-1 was performed by a leading, independent testing laboratory. Blood samples for 20 male Sprague Dawley rats (two groups of 10) are represented in the graph above taken at intervals of 2, 4, 6, 8, 12, 15, 30, 45, and 60 minutes, and at 2, 4 and 6 hours. Brain tissues were collected at 8 and 24 hours.
NIC-A21-1 Results
On October 5, 2021, Lexaria published results from its NIC-A21-1 in vivo trial of DehydraTECH-NICBZ (nicotine benzoate) delivered via oral pouch. The approach only required 2-4 minutes to deliver nicotine levels in blood plasma comparable to levels achieved at 45 minutes with concentration-matched controls. DehydraTECH-nicotine also reached statistically significant peak blood plasma levels up to 10-fold higher overall than controls (p=0.004) while still clearing from blood virtually as quickly as the controls.
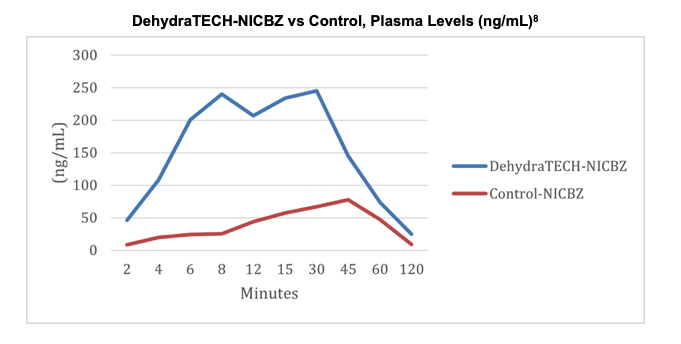
The data show that DehydraTECH-NICBZ was able to achieve higher peak and sustained levels of nicotine in blood plasma versus the control pouch, reaching peak delivery rates in as early as 8 minutes. Similarly, when comparing nicotine polacrilex, an alternate pouch formulation, DehydraTECH-NICPX was able to exceed control peak levels after just two minutes, and went on to reach peak plasma concentrations at an order of magnitude higher. The results are summarized as follows:
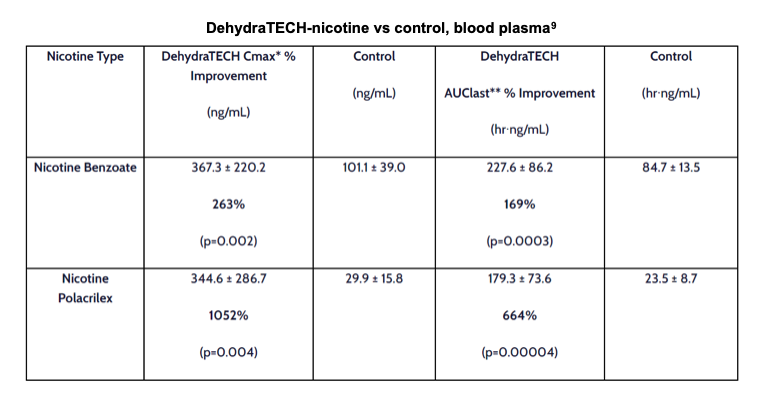
Results were all statistically significant, supporting further evaluation of the candidate. Lexaria plans to progress to a larger investigation in human volunteers of DehydraTECH-nicotine versus leading brands such as Zyn (Swedish Match) and ON! (Altria). Lexaria is currently in the design phase of this proposed human clinical study, which will be independently funded with existing capital. Details will be publicized as they become available.
Study NIC-A21-1 was performed by a leading, independent testing laboratory in 40 anesthetized male beagle dogs weighing roughly 8-14 Kg each. The dogs were divided into four groups of ten, and each had one of the four study test article nicotine pouches placed in their buccal space while laying on their side for 30 minutes. The pouches were uniformly squeezed gently every five minutes over this period and care was taken to ensure that saliva did not escape their mouths through the entire two-hour duration of the study observation period. Blood samples were taken at intervals of 0, 2, 4, 6, 8, 10, 15, 30, 45, 60 and 120 minutes. All dogs were well-treated, tolerated the pouches well and returned to full health following conclusion of the study.
Corporate Milestones (YTD)
➢ Announcement of 1-for-30 reverse split - January 2021
➢ Uplisting to Nasdaq Capital Market - January 2021
➢ Pricing and closing of $11M public offer - January 2021
➢ Al Reese, Jr. appointed to Board of Directors - January 2021
➢ CBD beverage shelf stability results - March 2021
➢ Gregory Downey appointed CFO - April 2021
➢ Issuance of warrants for 300,000 common shares - April 2021
➢ HYPER-H21-1 underway - April 2021
➢ First ever patent granted in India: DehydraTECH beverage - May 2021
➢ HYPER-H21-2 starts - June 2021
➢ 2021 Annual Meeting results - June 2021
➢ Voluntary delisting from CSE - July 2021
➢ First and second patents granted in Japan - July 2021
➢ HYPER-H21-2 dosing complete - July 2021
➢ Ibuprofen study results - 3Q:21
➢ Topline results from HYPER-H21-2 study - September 2021
➢ Results from nicotine animal study (canines) - September/October 2021
➢ THC (THC-A21-1) animal study results - 4Q:21
➢ Sildenafil/PDE5 inhibitor (PDE5-A21-1) animal study results - 4Q:21
➢ Launch of CBD HTN human study (HYPER-H21-3) - 4Q:21
Summary
We recently initiated on Lexaria, explaining its DehydraTECH technology platform and its wide applicability to enabling oral administration of products and therapies. DehydraTECH enhances bioavailability of active compounds via the digestive tract and topical administration and appears to be superior to other forms of administration including alternative types of oral delivery as supported by its preclinical studies.
In this report, we review Lexaria's progress on multiple fronts in the period since our initiation and also provide a video update with CEO Chris Bunka in a series of short videos (one, two, three). The updates include results from the in-human HYPER-H21-2 trial of DehydraTECH-CBD in hypertension, results from THC-A21-1 and NIC-A21-1 which exhibited DehydraTECH's ability to deliver substance via oral administration far more effectively than standard approaches, the commencement of EPIL-A21-1, and the nearing of completion for HYPER-H21-3. Lexaria also updates its trial plans for calendar year 2022, including the in-human HYPER-H21-4, as well as the in vivo HOR-A22-1, DEM-A22-1, RHEUM-A22-1 and DIAB-A22-1 in estrogen, dementia/Alzheimer's, rheumatoid disease, and diabetes, respectively.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.
________________________
2. van Hout, M.J., Dekkers, I.A., Westenberg, J.J. et al. Normal and reference values for cardiovascular magnetic resonance-based pulse wave velocity in the middle-aged general population. J Cardiovasc Magn Reson 23, 46 (2021). https://doi.org/10.1186/s12968-021-00739-y
3. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010 Mar 30;55(13):1318-27. doi: 10.1016/j.jacc.2009.10.061. PMID: 20338492.
4. Medical marijuana - Harvard Health
5. Lexaria's Technology Proven to Deliver Oral THC More Effectively :: Lexaria Bioscience Corp. (LEXX)
6. Lexaria's Technology Proven to Deliver Oral THC More Effectively :: Lexaria Bioscience Corp. (LEXX)
7. Lexaria's Technology Proven to Deliver Oral THC More Effectively :: Lexaria Bioscience Corp. (LEXX)







