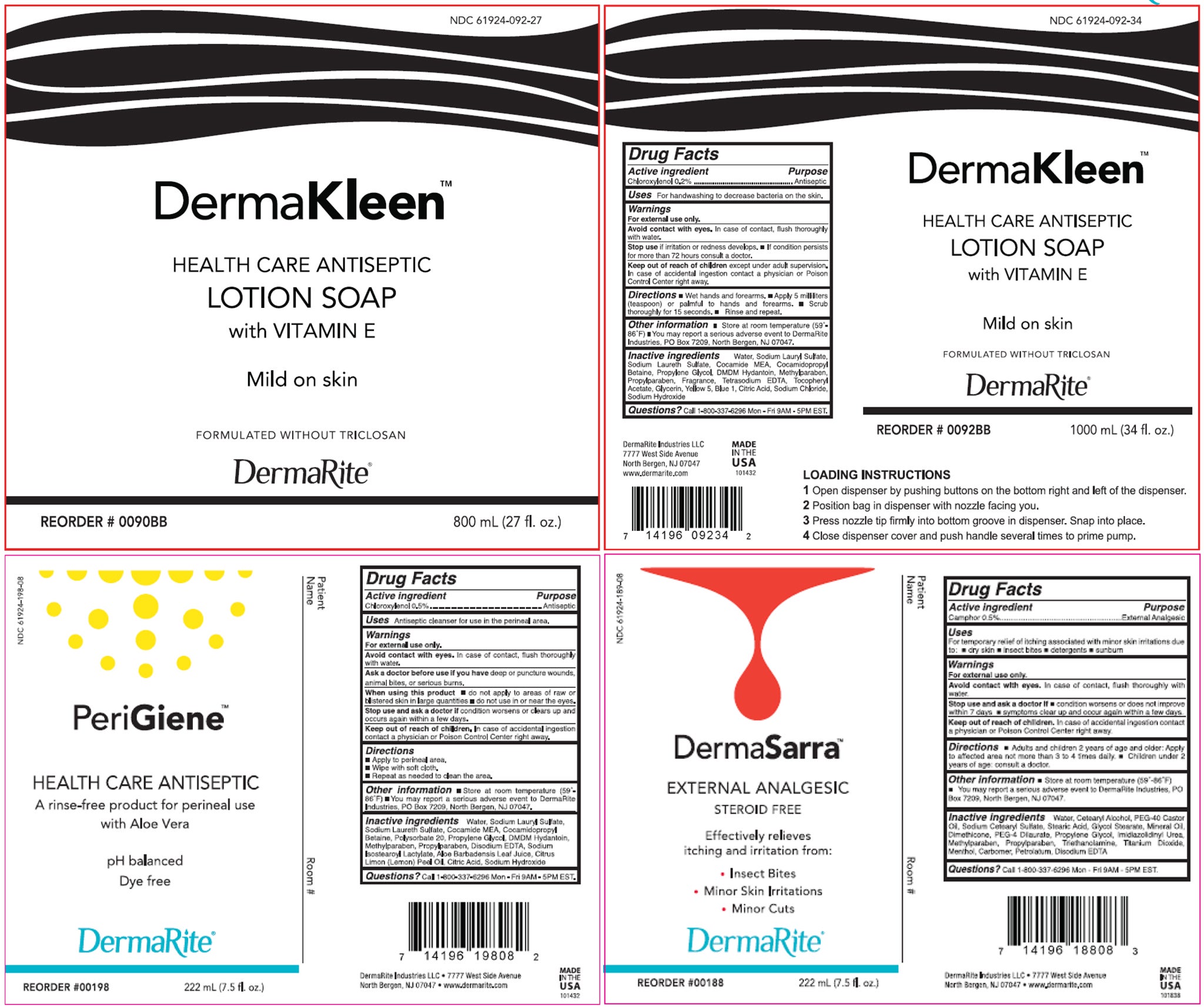
A medical hand soap brand has recalled four different types of its products over concerns of a potentially deadly bacteria.
DermaRite raised the alarm after it detected a microbial contamination, known as Burkholderia cepecia, in its soap products sold across the United States and Puerto Rico. Burkholderia Cepacia Complex – an antibiotic resistant bacteria that can cause infections in healthcare settings – can lead to serious and life-threatening infections, according to the U.S. Centers for Disease Control and Prevention.
The soaps are all over-the-counter antiseptic products that are used by physicians or healthcare professionals in settings such as hospitals and nursing homes. The contaminated products may be used by immunosuppressed individuals or by people attending to immunosuppressed individuals, the company said in a press release.
B. cepacia spreads from exposure to water, soil or aqueous (watery) environments, contact with contaminated surfaces, contact with contaminated equipment, and person-to-person transmission, more commonly in patients with cystic fibrosis, according to the CDC.
Healthy people who have small cuts on their skin and use of the soaps are more likely to suffer from local infections, they said. However, for immunocompromised individuals the infection is more likely to spread into the blood stream and lead to life-threatening sepsis.
Since the announcement on Friday, DermaRite has not received any reports of any “adverse events” related to the recall.
According to the CDC, B. cepacia germs can be resistant to antibiotics, making them difficult to treat.
Symptoms can range from no symptoms to serious respiratory infections, especially in patients with cystic fibrosis or other chronic lung disease, the CDC says. Other symptoms may include fever and fatigue.
Dermarite has recalled dozens of each product.
The four types include:
The full list of items in the recall are included on their website.
The soap brand said it had notified its distributors and customers by e-mail to immediately examine the stock available and destroy all the affected products.
To reduce the risk of infection, patients and caregivers should aim to keep their hands clean, particularly before and after caring for wounds or touching a medical device.
They should ask others to clean their hands before touching the patient or handling medical devices, avoid exposing wounds and indwelling medical devices (devices put in or connected to the patient's body) to nonsterile water, and allow healthcare staff to clean their room daily when in a healthcare setting, says the CDC.
If customers experience any adverse reactions after using the product, they should contact their physician or healthcare provider.
Adverse reactions or quality problems experienced with the use of any of the products may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Any customers who have questions relating to the recall should call Mary Goldberg at 973-569-9000 x104 Monday through Friday, 9:00 am – 5:00 pm EST or email voluntary.action@dermarite.com.







