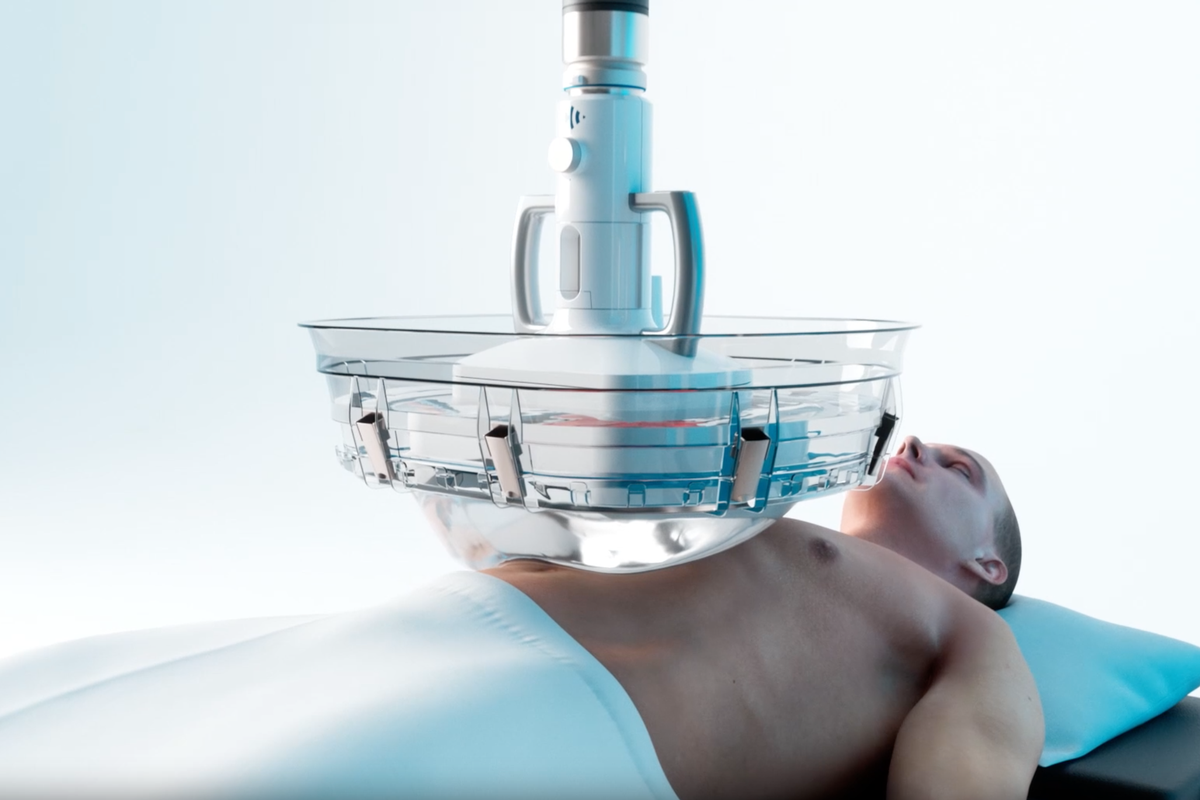
NHS patients will be the first in Europe to benefit from a pioneering new cancer treatment which uses ultrasound to destroy tumours.
The technology, known as histotripsy, offers a non-invasive treatment capable of breaking down liver cancer tissue without surgery, radiation or chemotherapy, with minimal damage to the surrounding organs, according to the Department for Health and Social Care (DHSC).
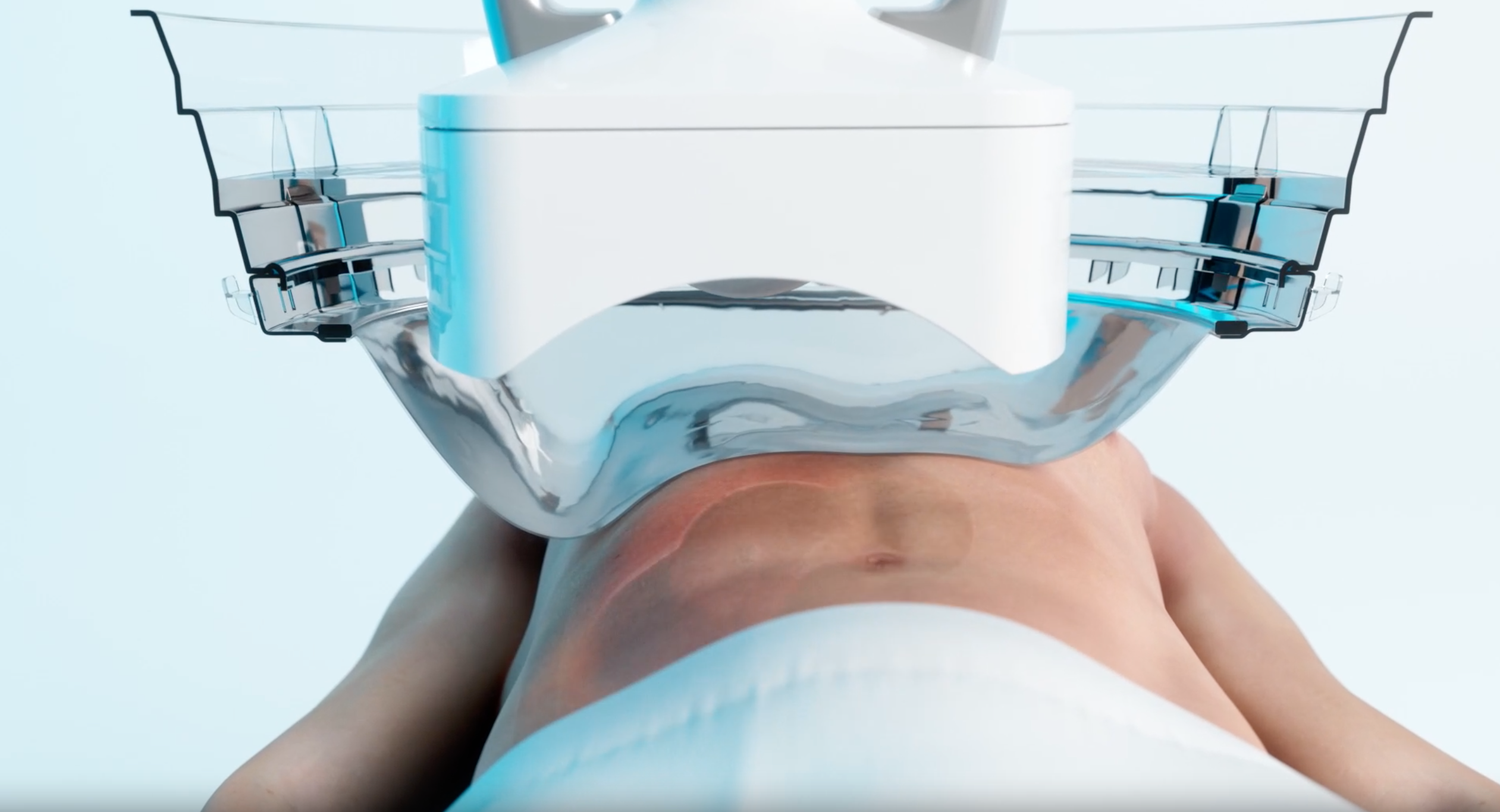
First approved by US regulators in October 2023, histotripsy uses pulsed sound waves to create “bubble clouds” from gases present in the targeted tissue and tumour.
When these bubbles pop, within microseconds, a shockwave is released which is capable of breaking up a mass and killing cancerous cells, while avoiding the harms of radiation and reducing the risks of bleeding, infection, and damage to surrounding non-targeted tissue.
Treatment is delivered via a single session – potentially taking just 30 minutes – with limited or no pain, a quick recovery, and can be performed as a day case, according to the DHSC.
The first NHS patients are set to be treated this summer at Addenbrooke’s Hospital in Cambridge, where the technology – which uses a device called an Edison System, created by US firm HistoSonics – is being debuted after a donation from the Li Ka Shing Foundation.
Cancer Research UK describes liver cancer as the 17th most common cancer in the UK and the 8th most frequent cause of cancer death. More than 8,000 people received a new diagnoses in 2022, with liver cancer incidence across the UK having surged by 42 per cent over the past decade, according to the British Liver Trust.
So far, more than 1,500 patients worldwide have received treatment using histotripsy, mainly in the United States – following approval by the US Food and Drug Administration in late 2023.
According to 12-month follow-up data from HistoSonics’ previous clinical trials, which involved patients for whom other treatments had been either unsuccessful or unavailable, overall survival rates after one year were 73.3 per cent for primary liver cancer, and 48.6 per cent for those with secondary tumours.
The paper, published in the Annals of Surgery journal in April, noted that both tumour control and survival rates were similar to those among other current treatments.
The UK’s new device is expected to be fully installed in Cambridge later this year, where it will be used initially to treat patients with primary and secondary liver tumours.
Wider research on how this could potentially treat tumours in other organs – such as the pancreas and kidneys – is underway.
The technology has been approved in the UK via the innovative devices access pathway, which aims to enable faster approvals of medical devices and treatments which evidence shows are safe, efficient and can fill unmet need.

“This is a strong example of smart, agile regulation in action,” said James Pound, of the Medicines and Healthcare products Regulatory Agency (MHRA). “It’s a major step forward for patients with liver cancer and shows how the UK can be a frontrunner in supporting responsible innovation that meets real clinical need.”
While “regulation is vital to protect patients”, the government is “slashing red tape, so game-changing new treatments reach the NHS front line quicker – transforming healthcare”, said health secretary Wes Streeting.
Hailing histotripsy as “an exciting new technology that will make a huge difference to patients”, Roland Sinker, chief executive of Cambridge University Hospitals, said: “By offering this non-invasive, more targeted treatment we can care for more people as outpatients and free up time for surgeons to treat more complex cases.
“The faster recovery times mean patients will be able to return to their normal lives more quickly, which will also reduce pressure on hospital beds, helping us ensure that patients are able to receive the right treatment at the right time.”
Fiona Carey, a kidney cancer patient who co-chairs Cambridge’s patient advisory group, added: “This is seriously good news. A new, non-invasive option to treat these cancers is very welcome indeed.
“For patients for whom ordinary surgery is no longer an option, this could make all the difference.”






