In 2019, Nenad Sestan and a team of researchers at Yale University announced the seemingly impossible: They had revived a pig’s brain after death, restoring cellular functions that, according to the laws of nature, should have been entirely snuffed out. Now, Sestan and his team are back, and this time, they reveal a way to partially revive all the organs in the pig’s body after death.
What’s new — In a paper published Wednesday in Nature, Sestan and his team at Yale University describe their technique using dead pigs that restored oxygen-starved tissues and organs, encouraging and even genetically influencing cells to repair themselves. This is the first time anyone has been able to restore organs in a body all at once.
These new findings could open the door for future innovations in organ transplantation, Nenad Sestan, the new study’s co-author and a neuroscientist at the Yale School of Medicine, told reporters at a press conference Tuesday.
In the U.S., over 105,000 people await a new organ. Not all of them may get one: Because there aren’t enough viable organs to go around, 17 people die each day as they wait, according to federal organ donation statistics. Genetically hacking animal organs for human transplant is one potential solution to the backlog, but preserving these tissues for use after death is difficult — Sestan’s technique may be the gamechanger.
While it’s too soon to say whether this innovation could help doctors counteract the effects of aging on the human body and enable humans to live longer, it may have more immediate clinical applications in reversing injury caused by poor blood flow, such as with strokes or heart attacks.
Here’s the background — To survive, our cells need a steady stream of oxygen. When that pipeline is cut off — when blood flow is restricted by injury or death — it leads to widespread system failures, and our cells stress out and die within minutes.
Reintroducing oxygen to a dead or dying organ may seem like an easy solution, but it’s not. When the heart stops beating, organs start to swell, explained David Andrijevic, the study’s co-author and also a neuroscientist at the Yale School of Medicine, to reporters on Tuesday. The swelling leads to collapsed blood vessels that block circulation and cause tissue damage, which clinicians call reperfusion or reoxygenation injury.
When it comes to organ donation, offsetting the deleterious effects of oxygen deprivation is key. The gold standard is to retrieve organs as quickly as possible after death and put them in what’s called static cold storage, which keeps the organs “alive” and viable by slowing down their metabolic needs.
An organ can’t be kept in storage indefinitely as it will lose its viability at some point. But scientists have devised technologies like ex vivo normothermic machine perfusion, where organs are kept in a “warm” state and are fed a continuous cocktail of blood and other nutrients.
Perfusing an organ outside a human body has so far been successful for reinstating single organs (and even transplanting them), not so much for an entire body since undoing systemic injury and damage has been a bit more challenging.
How they did it — The Yale researchers worked off an earlier experiment where they somewhat revived pig brains from slaughtered animals. The Lazarus-esque method dubbed BrainEx involved hooking up crucial blood vessels to a device that, for six hours, pumped out a specialized liquid containing a synthetic hemoglobin called Hemopure (which transports oxygen in red blood cells), nutrients like glucose, antibiotics, and other drugs that prevent blood clotting and cell death.
In their BrainEx experiment, the researchers found that neural tissue came back to life, not consciously but that the cells weren’t dying, they weren’t breaking down structurally, and operating as if they hadn’t just been dead.
Encouraged by their results, Andrijevic, Sestan, and the rest of their team adapted BrainEx for the entire body, what they call OrganEx. They took six dead female pigs, left the bodies for an hour after death to let nature take its course, and then pumped some of the pigs with the special liquid for six hours. To compare how effective OrganEx was to other techniques, the researchers had some of the pigs connected to an extracorporeal membrane oxygenation system (or ECMO), which essentially recirculates the pig’s own blood but with a fresh supply of oxygen.
Not only was OrganEx able to circulate oxygen and blood and reverse rigor mortis better than ECMO, tissues in organs in the heart, lungs, liver, kidneys, and pancreas remained intact, cells weren’t dying, and quite a number of cells were guzzling glucose with gusto, showing they were metabolically active.
OrganEx also appeared to prevent porcine cells from tripping inflammatory switches that lead to cell death, and activated genes involved in DNA repair and metabolism and suppressed those involved with death and injury.
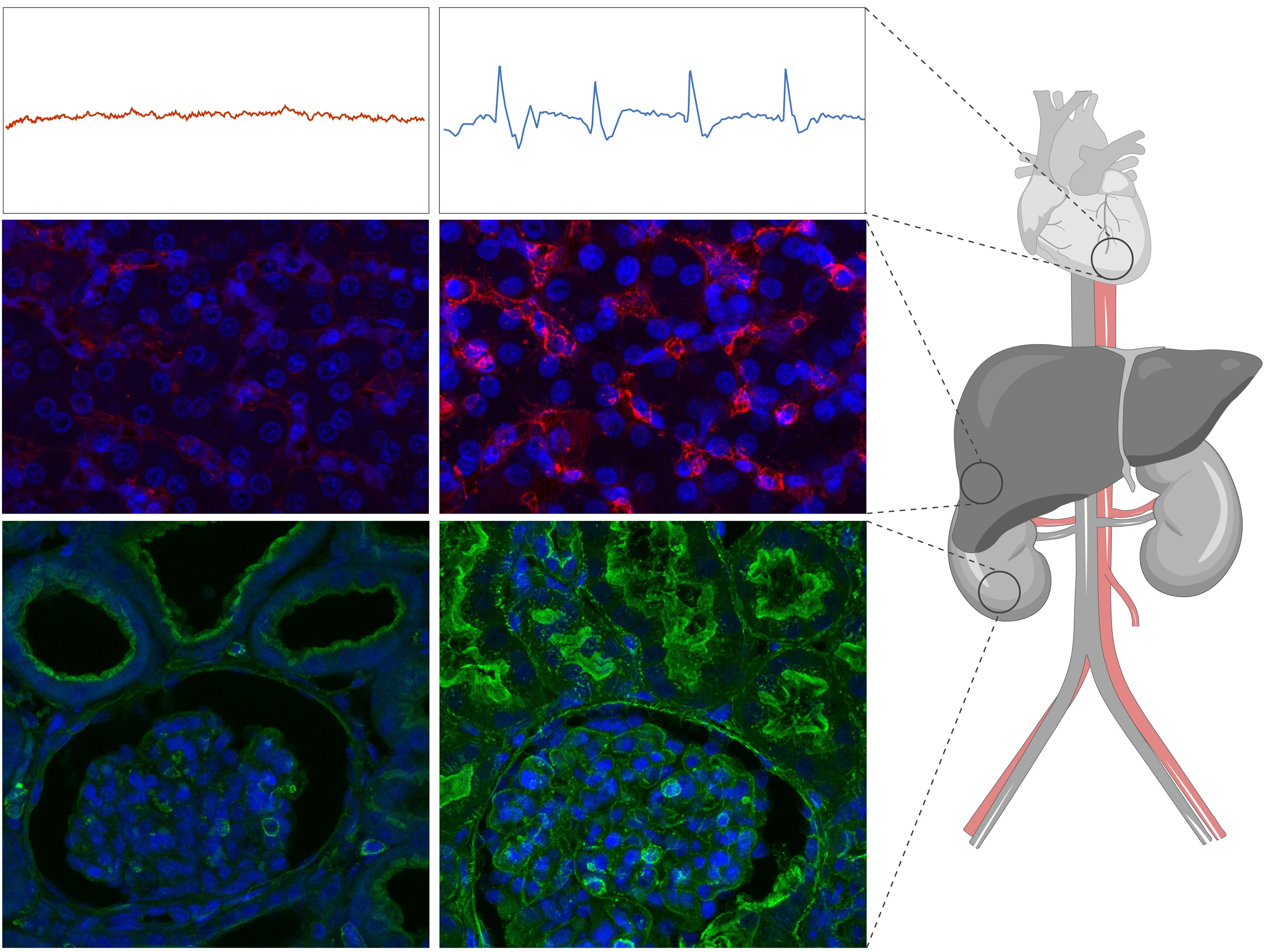
Digging into the details — Andrijevic said that what makes OrganEx work is a concert of modalities coming together, both pharmacological and technological.
“Why we think [OrganEx] works much better is that we have added several other pieces together to make it work,” he said.
“For example, it also has an artificial kidney system inside the circuit [and] perfusate, the special fluid circulating inside. Also, the whole system is supplemented with various sensors that can tell us in real-time the perfusion parameters so we can intervene [at any time]. We’ve also constructed a pulse generator that mimics the rhythm of the heart.”
A caveat to having such a complex system is whether all its components contribute to the benefits the researchers observed. It’s also unclear how enduring the treatment’s benefits are. Because of ethical regulations, the Yale researchers weren’t able to run longer studies on the pig bodies but they did take samples of brain tissue and found that even after two weeks of chilling in a Petri dish, the OrganEx-treated cells still appeared to be kicking.
What’s next — This proof-of-concept still needs more research done before it can be applied to humans, said Stephen Latham, a bioethicist at Yale’s Interdisciplinary Center for Bioethics involved in the study.
“We need to study a lot more detail, the degree to which damage [due to insufficient blood flow] is undone in different kinds of organs before it would even be close to thinking about trying an experiment like this on a human being,” he told reporters on Tuesday.
But once further research is done, the researchers see it possible that their tech could be used to reverse damage caused by interruptions in oxygen like heart attack, stroke, or other emergency settings. Most importantly, they hope their findings could one day up the supply and improve the quality of transplant organs.
“If this really works,” said Yale neuroscientist and study co-author Zvonimir Vrselja, “it would significantly impact organ availability in the future and save lives.”







