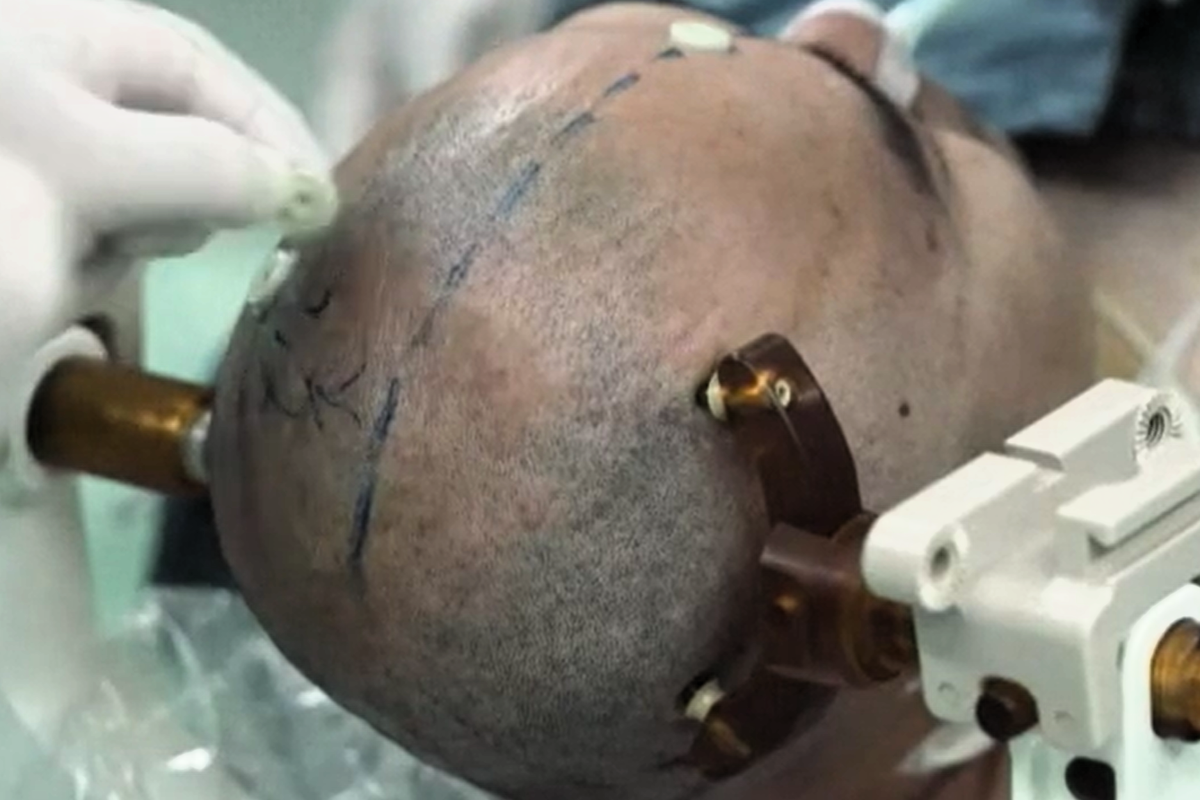
China has become the second country to launch clinical trials for invasive brain-computer interface (BCI) devices in humans.
The first brain chip was tested on a 37-year-old man who lost all four limbs in a high-voltage electrical accident, according to local reports, allowing him to play video games using only his mind.
The team now hope to develop the BCI to allow the patient to control a robotic arm or artificial intelligence agent.
The initial trial, which took place at the Center for Excellence in Brain Science and Intelligence (CEBSIT) at the Chinese Academy of Sciences, involved inserting tiny neural electrodes through a hole in the skull to read brain activity.
“The electrode is so soft that the force required to bend it is comparable to the interaction force between two neurons in the brain,” said Zhao Zhengtuo, a researcher at CEBSIT.
“This allows the electrode to coexist harmoniously with brain tissue over extended periods without triggering immune responses or rejection reactions.”

Research teams in the US are also testing invasive BCI devices on human patients, including Elon Musk’s Neuralink startup.
The tech billionaire has announced plans to implant millions of people’s brains with Neuralink chips over the next decade, following successful trials that saw participants control computers using their thoughts.
“If all goes well, there will be hundreds of people with Neuralinks within a few years, maybe tens of thousands within five years, millions within 10 years,” Mr Musk said last year.
Early Neuralink trials have focussed on people with quadriplegia, however Mr Musk claims the technology can be used to augment human intelligence and abilities.
Eventually, BCI devices could allow humans to merge with AI, according to the Neuralink boss, allowing people to compete with artificial general intelligence (AGI).
Neuralink recently raised $650 million in new funding to help expand its testing program globally and “expand what’s possible for humanity”.
China’s CEBSIT says that it expects its BCI to be ready for market by 2028, launching first as a medical device to improve the quality of life for amputees and people with spinal cord injuries.
The Shanghai-based lab claims its device is already smaller and more flexible than Neuralink’s BCI chip.







