Bismuth is an unusual element that we don't encounter much in everyday life. But this pretty, iridescent metal, found near the bottom of the periodic table, exhibits some extraordinary properties. Magnetic levitation — bismuth's ability to seemingly float between two magnets — is perhaps one of the most interesting. The repulsion between bismuth and the magnets is so strong, it causes the metal to levitate.
But why is bismuth so strongly repelled from magnets?
According to Eric Riesel, a magnetic materials chemist at MIT, the answer comes down to the type of magnetism exhibited by bismuth. Every material has magnetic properties, determined by a quantum property of the element's electrons known as spin. But, this spin can only point in two directions — up or down — and the combination of all the spins in a material define exactly what type of magnetism the element will exhibit.
"Most people are familiar with ferromagnets (permanent magnets) like iron, where the spins are all aligned with each other, but there are also anti-ferromagnets where the spins are pointed in opposite directions to each other," Riesel told Live Science.
However, there's also another pair of magnetic categories: paramagnetism and diamagnetism. "In paramagnets, when you apply a magnetic field, spins in that material will align with the field in proportion to its strength," he said. "Diamagnets apply a force in the opposite direction to the field, repelling it."
Related: Is it possible to reach absolute zero?
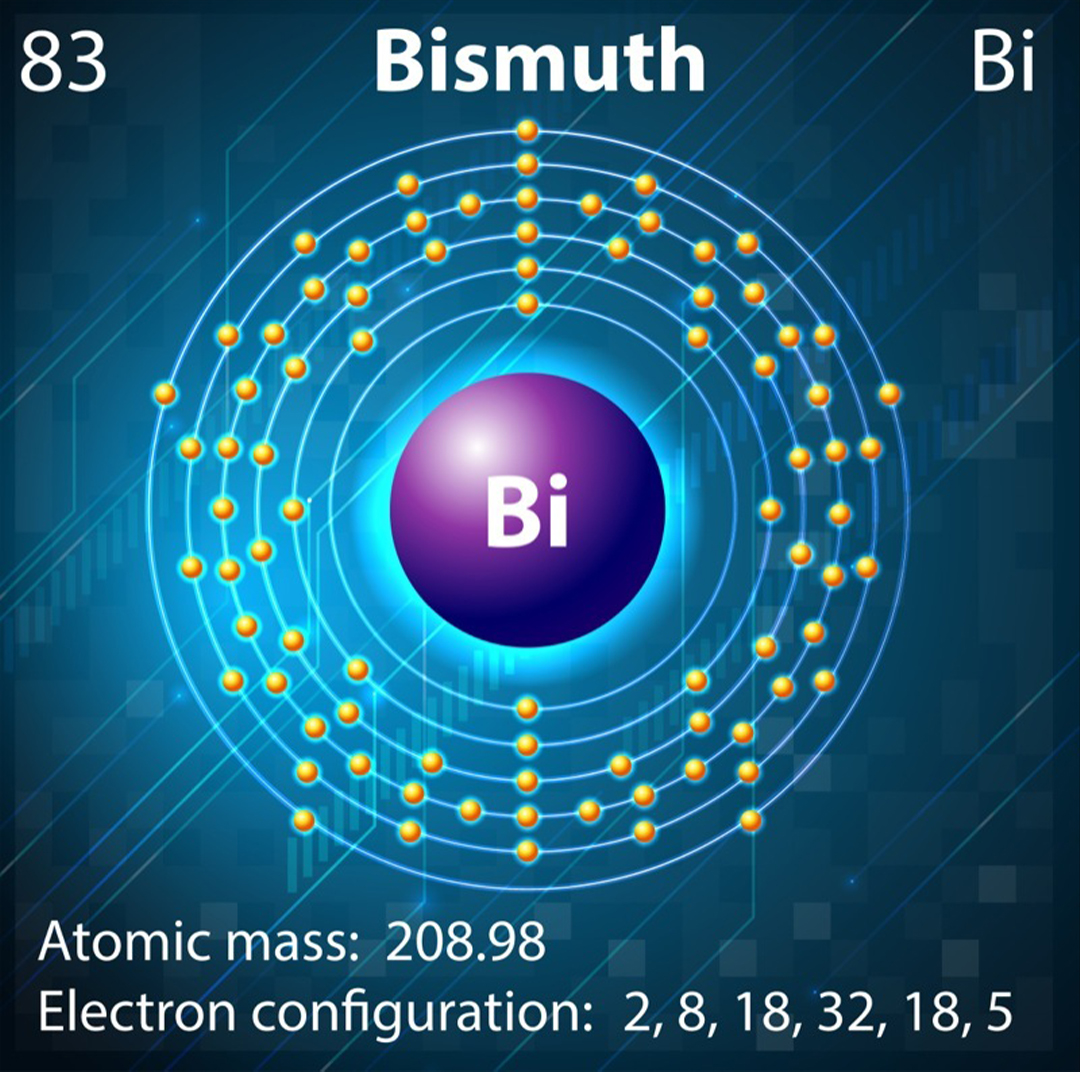
Bismuth is an example of a diamagnetic material, yet this is not the behavior we would expect from the element's electron configuration. The type of magnetism exhibited by a material depends on the arrangement of electrons and their corresponding spins. Electrons circle the nucleus in defined layers called shells, which are further subdivided into levels called the s, d, p and f orbitals.
Typically, diamagnetic materials have a closed shell structure. This means a particular group of orbitals are completely full and the electrons have been forced to pair, with one pointing up and the other down — essentially canceling out the spins. Conversely, paramagnetic materials usually have partially filled orbitals, meaning the electrons are unpaired and can align their spins in the same direction.
Bismuth is in Group 15 of the periodic table. The s, d and f orbitals are all full, but the p orbitals contain three out of a possible six electrons. So bismuth has partially filled orbitals and should behave as a paramagnet. However, its position in row six of the periodic table means bismuth also possesses some unusual heavy-atom properties.
"Chemical elements found after the f-block in the periodic table have their outermost electrons orbiting the nucleus at speeds that are significant fractions of the speed of light," said Ira Martyniak, also a magnetic materials chemist at MIT. "The direct relativistic effect makes the 6s and 6p orbitals contract and reside closer to the nucleus, which gives rise to anomalous physical and chemical characteristics."
These relativistic effects are responsible for many of bismuth's surprising properties, such as its unconventional superconductivity, its very low melting point (520.7 degrees Fahrenheit, or 271.5 degrees Celsius) and the unusual shape of its crystals. The unexpected diamagnetism is no exception.
"Even though bismuth has the unpaired electrons in its 6p orbital, because of relativistic contraction of the 6s and 6p levels, the paramagnetism stemming from the 6p electrons is suppressed and the behavior of bismuth is largely dominated by the closed shells and large size of the atom, leading to strong diamagnetism," Martyniak told Live Science.
Diamagnetic materials have lots of valuable applications, including electromagnetic induction in copper coils (used to generate electricity) and the aluminum tracks of high-speed maglev trains. Bismuth itself is too heavy to be a practical material for general use, but its potent diamagnetism means it is now a common component in superconductors and quantum computing.






